Allergic rhinitis (AR) is a common medical condition affecting up to 40% of the general population. A type 2 immunity determines eosinophilic inflammation that, in turn, elicits typical nasal symptoms. Type 2 immunity is eminently characterized by polarization of innate and adaptative B and T cells, increased production of type 2 cytokines, including interleukin-4 (IL-4), IL-5, and IL-13, and impaired function of allergen-specific T regulatory cells (Tregs). This immunologic derangement promotes allergic inflammation, characterized by an abundant eosinophilic infiltrate and the presence of mast cells. The mast cells are activated by allergen exposure and release pro-inflammatory mediators, including histamine. These mediators interact with specific receptors and, consequently, are responsible for the appearance of typical AR symptoms: nasal itching, sneezing, rhinorrhea, and nasal congestion.
- oral probiotics
- allergic rhinitis
- dysbiosis
- inflammation
- immunity
1. The Rationale for Probiotic Use in Allergic Rhinitis Management
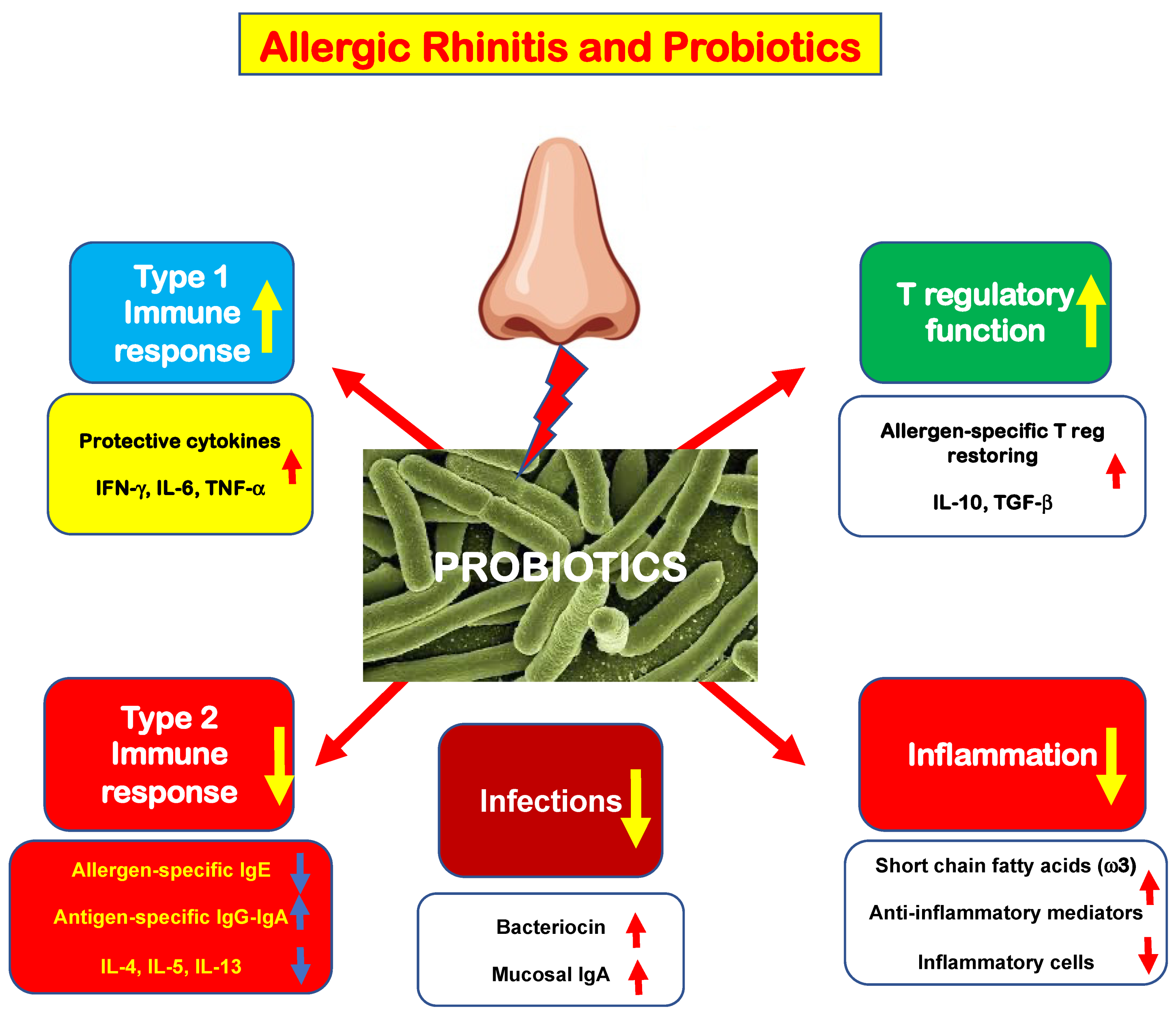
2. New Evidence in the Literature
This entry is adapted from the peer-reviewed paper 10.3390/allergies2030011
References
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260.
- Haahtela, T. A biodiversity hypothesis. Allergy 2019, 74, 1445–1456.
- Convention on Biological Diversity. 1992. Available online: www.biodiv.org/convention (accessed on 4 July 2022).
- Hufnagl, K.; Pali-Schoell, I.; Roth-Walter, F.; Jensen-Jarolim, E. Dysbiosis of the gut and lung microbiome has a role in asthma. Sem. Immunopathol. 2020, 42, 75–93.
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients 2020, 12, 946.
- Kang, Y.B.; Cai, Y.; Zhang, H. Gut microbiota and allergy/asthma: From pathogenesis to new therapeutic strategies. Allergol. Immunopathol. (Madr) 2017, 45, 305–309.
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850.
- Matsubara, A.; Nomura, A.; Yamaguchi, T. The relationship between allergic rhinitis and gut microbiota. Aerugi. 2022, 71, 191–194.
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.D.C.; Regal, P.; Miranda, J.M. Probiotics as a Possible Strategy for the Prevention and Treatment of Allergies. A Narrative Review. Foods 2021, 10, 701.
- FAO/WHO. Expert Consultation Health and Nutrition Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Available online: http://isappscience.org/wp-content/uploads/2015/12/FAO-WHO-2001-ProbioticsReport.pdf (accessed on 4 July 2022).
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, 549–566.
- Wang, H.T.; Anvari, S.; Anagnostou, K. The role of probiotics in preventing allergic disease. Children 2019, 6, 24.
- Balta, I.; Butucel, E.; Mohylyuk, V.; Criste, A.; Dezmirean, D.N.; Stef, L.; Pet, I.; Corcionivoschi, N. Novel insights into the role of probiotics in respiratory infections, allergies, cancer, and neurological abnormalities. Disease 2021, 9, 60.
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutrit. Metabol. 2019, 74, 115–124.
- Al Nabhani, Z.; Eberl, G. Imprinting of the immune system by the microbiota early in life. Mucosal Immunol. 2020, 13, 183–189.
- Huang, J.; Zhang, J.; Wang, X.; Jin, Z.; Zhang, P.; Su, H.; Sun, X. Effect of probiotics on respiratory tract allergic disease and gut microbiota. Front. Nutr. 2022, 9, 821900.
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386.
- Lin, C.; Lin, Y.; Zhang, H.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Intestinal “Infant-Type” Bifidobacteria mediate immune system development in the first 1000 days of life. Nutrients 2022, 14, 1498.
- Das, R.R.; Naik, S.S.; Singh, M. Probiotics as additives on therapy in allergic airway diseases: A systematic review of benefits and risks. BioMed Res. Int. 2013, 2013, 231979.
- Guvenc, I.A.; Bayar Muluk, N.; Mutlu, F.S.; Eski, E.; Altintoprak, N.; Oktemer, T.; Cingi, C. Do probiotics have a role in the treatment of allergic rhinitis? A comprehensive systematic review and meta-analysis. Am. J. Rhinol. Allergy. 2016, 30, 157–175.
- Lin, J.; Zhang, Y.; He, C.; Dai, J. Probiotics supplementation in children with asthma: A systematic review and meta-analysis. J. Ped. Child Health 2018, 54, 953–961.
- Du, X.; Wang, L.; Wu, S.; Yuan, L.; Tang, S.; Xiang, Y.; Qu, X.; Liu, H.; Qin, X.; Liu, C. Efficacy of probiotic supplementary therapy for asthma, allergic rhinitis, and wheeze: A meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2019, 40, 250–260.
- Wei, X.; Jiang, P.; Liu, J.; Sun, R.; Zhu, L. Association between probiotic supplementation and asthma incidence in infants: A meta-analysis of randomized controlled trials. J. Asthma 2020, 57, 167–178.
- Meirlaen, L.; Levy, E.I.; Vandenplas, Y. Prevention and management with pro-, pre- and synbiotics in children with asthma and allergic rhinitis: A narrative review. Nutrients 2021, 13, 934.
- Anania, C.; Di Marino, V.P.; Olivero, F.; De Canditiis, D.; Brindisi, G.; Iannilli, F.; De Castro, G.; Zicari, A.M.; Duse, M. Treatment with a Probiotic Mixture Containing Bifidobacterium animalis Subsp. Lactis BB12 and Enterococcus faecium L3 for the Prevention of Allergic Rhinitis Symptoms in Children: A Randomized Controlled Trial. Nutrients 2021, 13, 1315.
- Torre, E.; Sola, D.; Caramaschi, A.; Mignone, F.; Bona, E.; Fallarini, S. A Pilot Study on Clinical Scores, Immune Cell Modulation, and Microbiota Composition in Allergic Patients with Rhinitis and Asthma Treated with a Probiotic Preparation. Int. Arch. Allergy Immunol. 2022, 183, 186–200.
- Mårtensson, A.; Nordström, F.U.; Cervin-Hoberg, C.; Lindstedt, M.; Sakellariou, C.; Cervin, A.; Greiff, L. Nasal administration of a probiotic assemblage in allergic rhinitis: A randomised placebo-controlled crossover trial. Clin. Exp. Allergy. 2022, 52, 774–783.
- Ried, K.; Travica, N.; Paye, Y.; Sali, A. Effects of a Probiotic Formulation on Seasonal Allergic Rhinitis in Adults-A Randomized Double-Blind Placebo-Controlled Trial: The Probiotics for Hay Fever Trial. Front. Nutr. 2022, 9, 887978.
- Jakubczyk, D.; Górska, S. Impact of Probiotic Bacteria on Respiratory Allergy Disorders. Front. Microbiol. 2021, 12, 688137.
- Ciprandi, G.; Schiavetti, I.; Cioffi, L.; Pane, M.; Drago, L. The Probiotics in Pediatric Asthma Management (PROPAM) study: A Post Hoc analysis in allergic children. Ann. Allergy Asthma Immunol. 2022, in press.
- Ciprandi, G.; Tosca, M.A. Probiotics in children with asthma. Children 2022, 9, 978.
