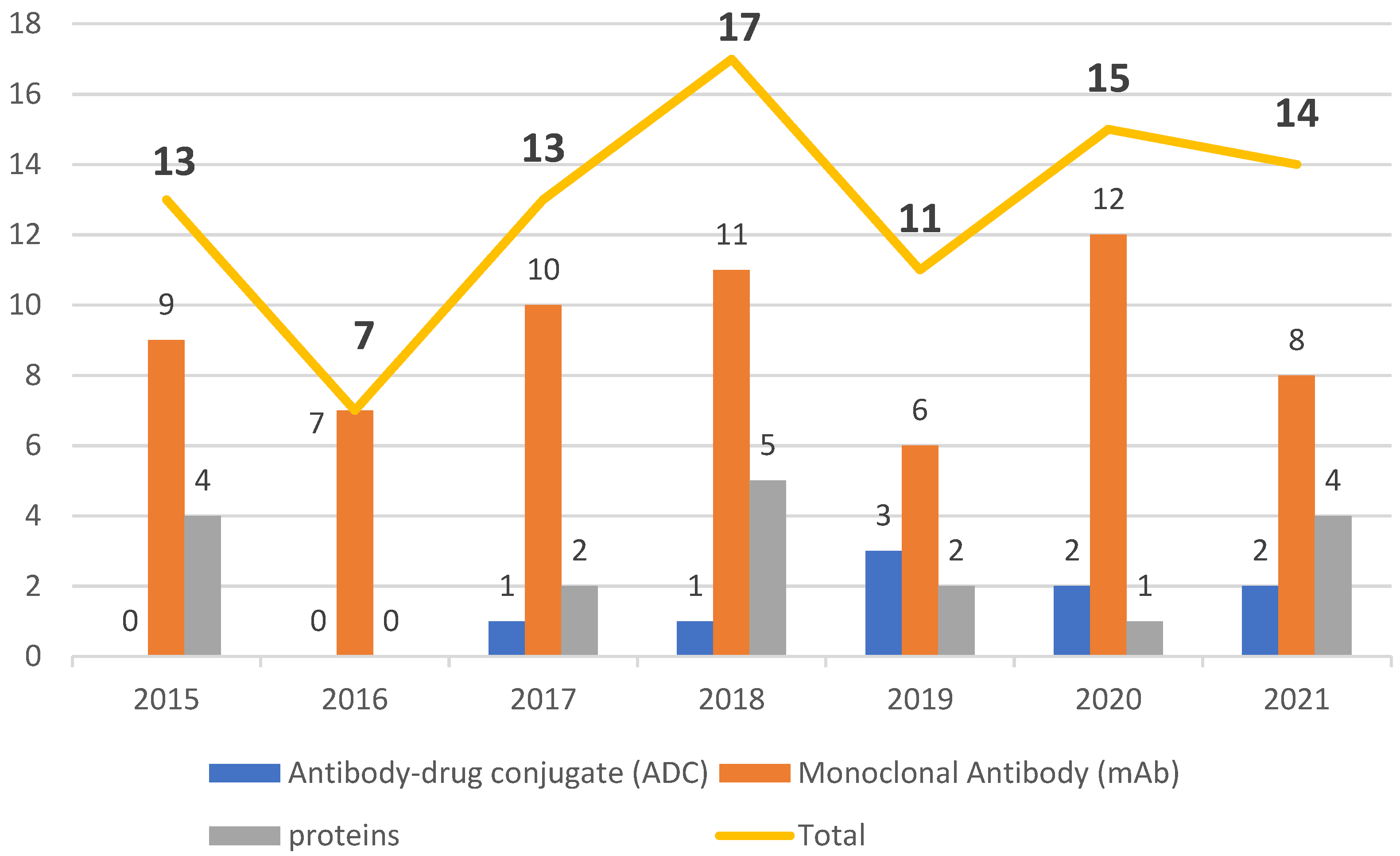
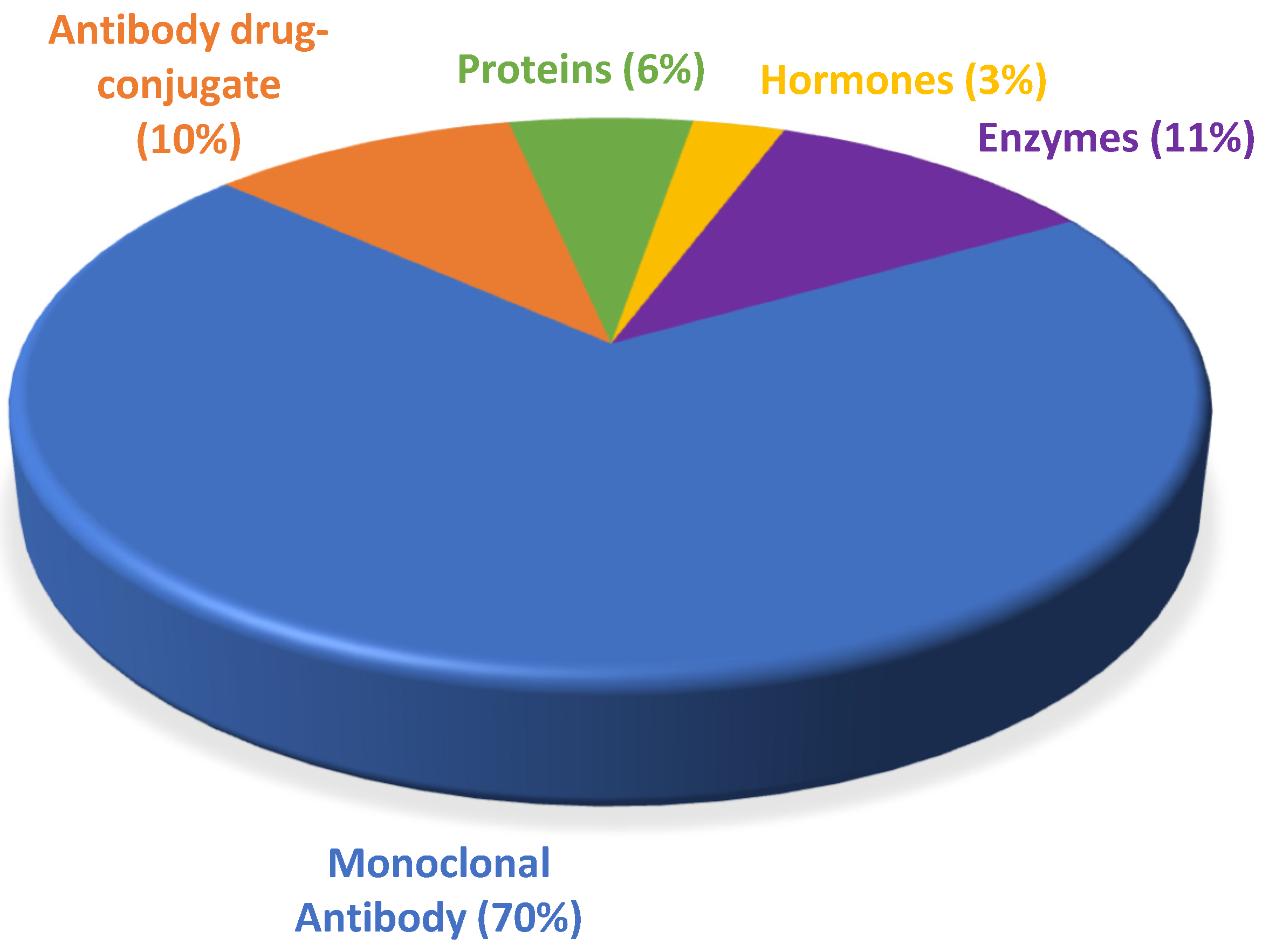
Despite belonging to a relatively new class of pharmaceuticals, biological drugs have been used since the 1980s, when they brought about a breakthrough in the treatment of chronic diseases, especially cancer. They conquered a large space in the pipeline of the pharmaceutical industry and boosted the innovation portfolio and arsenal of therapeutic compounds available. From 2015 to 2021, the number of drugs included in this class grew over this period, totaling 90 approvals, with an average of 13 authorizations per year.
- Food and Drug Administration
- FDA approvals
- monoclonal antibody
- antibody–drug conjugate
- biological drugs
1. Introduction
2. Timeline for FDA-Approved Biological Drugs
3. Therapeutic Indications
3.1. Cancer
3.2. Mechanisms of Action and Therapeutic Indications of ADCs and mAbs for Cancer
3.2.1. mAbs for Cancer
3.2.2. Antibody–Drug Conjugates
4. Autoimmune Diseases
The biologics for autoimmune diseases (psoriasis, plaque psoriasis, psoriatic arthritis, multiple sclerosis, myasthenia gravis, lupus erythematosus, rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and neuromyelitis optic spectrum disorder) in the period of interest included 13 biologics, 12 of which were mAbs, and a new class of biological, namely an antibody fragment (Efgartigimod alfa VyvgartTM), which is detailed in Table 1.
| Active Ingredient and Trade Name | mAb Class | Targets/Mechanism of Action | Original Approval Date |
Manufacturer | Therapeutic Indication |
|---|---|---|---|---|---|
| Cosentyx™ (Secukinumab) [6][36] |
Human | IL-17A inhibitor | 2015 | Novartis Pharmaceuticals | Plaque psoriasis, Psa, and AS |
| Zinbryta™ (daclizumab) [37][38] | Humanized | IL-2R inhibitor | 2016 | Biogen Inc | Multiple sclerosis |
| Taltz™ (ixekizumab) [37][39] | Humanized | IL-17A inhibitor | 2016 | Eli Lilly and Company | Plaque psoriasis and Psa |
| Tremfya™ (guselkumab) [8][40] | Human | IL-23 and IL-17A inhibitor | 2017 | Janssen Biotech, Inc | Plaque psoriasis |
| Ocrevus™ (Ocrezilumab) [41][42] | Humanized | Anti-CD-20 | 2017 | Genentech, Inc | Multiple sclerosis |
| Kevzara™ (sarilumab) [41][43] | Human | IL-6 inhibitor | 2017 | Sanofi-Aventis U.S LLC | Rheumatoid arthritis |
| Siliq™ (brodalumab) [41][44] | Human | IL-17A, IL-17F, and other IL-17 isoform inhibitors | 2017 | Valeant Pharmaceuticals Luxembourg S.à.r.l | Plaque psoriasis |
| Ilumya™ (tildrakizumab) [45][46] | Humanized | IL 23p19 | 2018 | Sun Pharma Global FZE | Plaque psoriasis |
| Skyrizi™ (risankizumab) [47][48] | Humanized | IL-23p19 inhibitor | 2019 | AbbVie Inc. | Plaque psoriasis and Psa |
| Uplizna™ (inebilizumab) [49][50] |
Humanized | Depletes CD-19 | 2020 | Horizon Therapeutics Ireland DAC | NMOSD |
| Enspryng™ (satralizumab) [11][51][52] |
Humanized | Anti-IL -6R | 2020 | Genentech, Inc. | NMOSD |
| Saphnelo™ (anifrolumab) [2][53] |
Human | Blocks the action of type 1 interferon receptor | 2021 | AstraZeneca AB | Lupus erythematosus |
| Vyvgart™ (efgartigimod alfa) [2][54] |
Human monoclonal ARGX-113 fc fragment | Neonatal Fc receptor antagonist | 2021 | Argenx BV | Generalized myasthenia gravis |
Of the approvals of autoimmune biopharmaceuticals from 2015 to 2021, six are indicated for psoriasis, plaque psoriasis, and psoriatic arthritis. Brodalumab Siliq™ is indicated for moderate to severe plaque psoriasis [44]. While this drug acts by antagonizing the IL-17A Receptor, Cosentyx™ and Taltz™ antagonize the pro-inflammatory cytokine IL-17A, which plays a role in psoriasis and Psa [36][39]. Guselkumab Tremfya™, used for the treatment of psoriasis and Psa, is an antibody that blocks the activity of two interleukins (IL-23, IL-17A) that are overexpressed in these diseases [42]. Tildrakizumab Ilumya™ is an IgG1 antibody that selectively binds to interleukin-23-p19 (IL-23A p19) [46] and, through the same mechanism, Risankizumab Syrizi™ also binds to the same p19 subunit of this interleukin. In some countries, there are trials underway to evaluate Risankizumab for the treatment of Crohn’s disease and ulcerative colitis [48][55][56].
5. Other Therapeutic Indications
| Active Ingredient and Trade Name | mAb Class | Target/Mechanism of Action | Original Approval Date | Manufacturer |
|---|---|---|---|---|
| Emgality™ (Galcanezumab) [45][60] | Humanized | CGRP antagonist | 2018 | Eli Lilly and Company |
| Ajovy™ (Fremanezumab) [45][59] | Humanized | CGRP antagonist | 2018 | Teva Branded Pharmaceutical Products R&D, Inc. |
| Aimovig™ (Erenumab) [45][60] | Human | CGRPR antagonist | 2018 | Amgen, Inc. |
| Vyepti™ (Eptinezumab) [11][57] | Humanized | CGRP antagonist | 2020 | Lundbeck Seattle Pharmaceuticals, Inc. |
| Active Ingredient and Trade Name | mAb Class | Target/Mechanism of Action | Original Approval Date | Manufacturer |
|---|---|---|---|---|
| Nucala™ (Mepolizumab) [6][64] |
Humanized | IL-5 | 2015 | GlaxoSmithKline LLC |
| Cinqair™ (Reslizumab) [37][65] |
Humanized | IL-5 | 2016 | Teva Respiratory LLC |
| Fasenra™ (Benralizumab) [41][66] |
Humanized | IL-5R-α | 2017 | AstraZeneca AB |
| Dupixent™ (Dupilumab) [41][61] |
Human | IL-4R-α | 2017 | Regeneron Pharmaceuticals, Inc. |
| Tezsipire™ (Tezepelumab) [2][63] |
Human | Blocks TSLP | 2021 | AstraZeneca AB |
6. Conclusions
The period 2015 to 2021 witnessed a growth in FDA approval of biologicals in general, with mAbs being the class with the greatest presence. During this period, the number of authorizations of biopharmaceuticals remained in the double figures, except in 2016, when only seven were given the green light. The years 2020 and 2021 did not show considerable variation, with one less biological being approved in 2021 than in 2020, while 2018 was the year with the highest number of approvals. Of note, even in the midst of the COVID-19 pandemic, the potential for these therapies to receive approval remained steady.
Biological medicines show high selectivity and high versatility and are therefore valuable. Their versatility is reflected in indications that range from the treatment of chronic or rare diseases to more aesthetic purposes such as the treatment of frown lines. These drugs offer great potential to be exploited for other therapeutic indications beyond what they were initially authorized for. In this regard, they offer a solid starting point from which to explore their capacity in clinical trials. For example, over the years, new applications have been discovered for Adalimumab HumiraTM, and today this drug has more than ten therapeutic indications listed in the directions of use [73]. Daratumumab DarzalexTM is also undergoing evaluation for other types of cancer, including refractory or relapsed non-Hodgkin’s Lymphoma [12]. mAbs can also be conjugated to toxins or drugs without compromising healthy tissues around the target fragment or at least minimizing effects in other tissues [74].
Between 2015 and 2021, in addition to the increase in the number of drug approvals, several breakthroughs and innovations took place, such as Aducanumab AduhelmTM, although still controversial, and also Tagraxofusp ElzonrisTM, which the FDA granted the status of Orphan Drug to treat rare diseases. In 2021, we witnessed the authorization of a different class of biological, Efgartigimod alfa VyvgartTM, an antibody fragment that also has Orphan Drug status [54], and the bispecific antibody approved within the period of interest HemlibraTM. Of note only two bispecific antibodies were approved in the period of interest HemlibraTM and RybrevantTM.
However, one of the great challenges for the development of biopharmaceuticals is the high technology required to produce these drugs, which makes them very expensive. We believe that, in the near future, this class of drugs will become increasingly accessible and new drugs will be developed. Moreover, more biosimilars will become accessible thanks to the development of new technologies that will impact production. These advancements will make these drugs increasingly more profitable and less expensive, which in turn will widen the accessibility of biological therapies, thereby expanding the therapeutic arsenal and transforming the management of diseases for which no treatment is available or diseases for which current treatments are not effective.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10092325
References
- Wu, A.C.; Fuhlbrigge, A.L.; Robayo, M.A.; Shaker, M. Cost-Effectiveness of Biologics for Allergic Diseases. J. Allergy Clin. Immunol. Pract. 2021, 9, 1107–1117.e2.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2021. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2022, 27, 1075.
- Batta, A.; Kalra, B.; Khirasaria, R. Trends in FDA Drug Approvals over Last 2 Decades: An Observational Study. J. Family Med. Prim. Care 2020, 9, 105.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2014. Available online: https://www.fdanews.com/ext/resources/files/01-15/01-02-15-Drug-Approvals-2014.pdf?1520892896 (accessed on 7 September 2022).
- U.S. Food And Drug Administration FDA Grants Accelerated Approval for Alzheimer’s Drug. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug#:~:text=Today%2C%20the%20U.S.%20Food%20and,disease%20affecting%206.2%20million%20Americans (accessed on 7 September 2022).
- U.S. Food and Drug Administration Novel Drug Approvals for 2015. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015 (accessed on 7 September 2022).
- Torre, B.; Albericio, F. The Pharmaceutical Industry in 2016. An Analysis of FDA Drug Approvals from a Perspective of the Molecule Type. Molecules 2017, 22, 368.
- de la Torre, B.; Albericio, F. The Pharmaceutical Industry in 2017. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2018, 23, 533.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2018. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2019, 24, 809.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2019. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2020, 25, 745.
- de la Torre, B.G.; Albericio, F. The Pharmaceutical Industry in 2020. An Analysis of FDA Drug Approvals from the Perspective of Molecules. Molecules 2021, 26, 627.
- McKeage, K. Daratumumab: First Global Approval. Drugs 2016, 76, 275–281.
- Dhillon, S. Isatuximab: First Approval. Drugs 2020, 80, 905–912.
- Sanchez, L.; Richter, J.; Cho, H.J.; Jagannath, S.; Madduri, D.; Parekh, S.; Richard, S.; Tam, L.; Verina, D.; Chari, A. Subcutaneous Daratumumab and Hyaluronidase-Fihj in Newly Diagnosed or Relapsed/Refractory Multiple Myeloma. Ther. Adv. Hematol. 2021, 12, 204062072098707.
- Gao, J.J.; Osgood, C.L.; Gong, Y.; Zhang, H.; Bloomquist, E.W.; Jiang, X.; Qiu, J.; Yu, J.; Song, P.; Rahman, N.A.; et al. FDA Approval Summary: Pertuzumab, Trastuzumab, and Hyaluronidase–Zzxf Injection for Subcutaneous Use in Patients with HER2-Positive Breast Cancer. Clin. Cancer Res. 2021, 27, 2126–2129.
- Melaragno, A. Rituximab/Hyaluronidase (Rituxan HycelaTM). Oncol. Times 2017, 39, 18.
- Keam, S.J. Trastuzumab Deruxtecan: First Approval. Drugs 2020, 80, 501–508.
- Markham, A. Margetuximab: First Approval. Drugs 2021, 81, 599–604.
- Shirley, M. Olaratumab: First Global Approval. Drugs 2017, 77, 107–112.
- Syed, Y.Y. Durvalumab: First Global Approval. Drugs 2017, 77, 1369–1376.
- Markham, A. Atezolizumab: First Global Approval. Drugs 2016, 76, 1227–1232.
- Kim, E.S. Avelumab: First Global Approval. Drugs 2017, 77, 929–937.
- Garnock-Jones, K.P. Necitumumab: First Global Approval. Drugs 2016, 76, 283–289.
- Syed, Y.Y. Amivantamab: First Approval. Drugs 2021, 81, 1349–1353.
- Garber, K. No Added Sugar: Antibody Makers Find an Upside to “No Fucose”. Nat. Biotechnol. 2018, 36, 1025–1026.
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927.
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Eng. J. Medic. 2021, 384, 1125–1135.
- Markham, A. Tisotumab Vedotin: First Approval. Drugs 2021, 81, 2141–2147.
- Deeks, E.D. Polatuzumab Vedotin: First Global Approval. Drugs 2019, 79, 1467–1475.
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025.
- Lee, A. Loncastuximab Tesirine: First Approval. Drugs 2021, 81, 1229–1233.
- Lamb, Y.N. Inotuzumab Ozogamicin: First Global Approval. Drugs 2017, 77, 1603–1610.
- Heo, Y.-A.; Syed, Y.Y. Subcutaneous Trastuzumab: A Review in HER2-Positive Breast Cancer. Target. Oncol. 2019, 14, 749–758.
- Markham, A. Belantamab Mafodotin: First Approval. Drugs 2020, 80, 1607–1613.
- Dhillon, S. Moxetumomab Pasudotox: First Global Approval. Drugs 2018, 78, 1763–1767.
- Blair, H.A. Secukinumab: A Review in Psoriatic Arthritis. Drugs 2021, 81, 483–494.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2016. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2016 (accessed on 7 September 2022).
- Cohan, S.; Lucassen, E.; Romba, M.; Linch, S. Daclizumab: Mechanisms of Action, Therapeutic Efficacy, Adverse Events and Its Uncovering the Potential Role of Innate Immune System Recruitment as a Treatment Strategy for Relapsing Multiple Sclerosis. Biomedicines 2019, 7, 18.
- Georgakopoulos, J.R.; Phung, M.; Ighani, A.; Yeung, J. Ixekizumab (Interleukin 17A Antagonist): 12-Week Efficacy and Safety Outcomes in Real-World Clinical Practice. J. Cutan Med. Surg. 2019, 23, 174–177.
- Markham, A. Guselkumab: First Global Approval. Drugs 2017, 77, 1487–1492.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2017. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2017 (accessed on 7 September 2022).
- Frampton, J.E. Ocrelizumab: First Global Approval. Drugs 2017, 77, 1035–1041.
- Scott, L.J. Sarilumab: First Global Approval. Drugs 2017, 77, 705–712.
- Puig, L. Brodalumab: The First Anti-IL-17 Receptor Agent for Psoriasis. Drugs Today 2017, 53, 283.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2018. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2018 (accessed on 7 September 2022).
- Markham, A. Tildrakizumab: First Global Approval. Drugs 2018, 78, 845–849.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2019. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed on 7 September 2022).
- McKeage, K.; Duggan, S. Risankizumab: First Global Approval. Drugs 2019, 79, 893–900.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2020. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed on 7 September 2022).
- Frampton, J.E. Inebilizumab: First Approval. Drugs 2020, 80, 1259–1264.
- Heo, Y.-A. Satralizumab: First Approval. Drugs 2020, 80, 1477–1482.
- Yamamura, T.; Kleiter, I.; Fujihara, K.; Palace, J.; Greenberg, B.; Zakrzewska-Pniewska, B.; Patti, F.; Tsai, C.-P.; Saiz, A.; Yamazaki, H.; et al. Trial of Satralizumab in Neuromyelitis Optica Spectrum Disorder. N. Eng. J. Med. 2019, 381, 2114–2124.
- Deeks, E.D. Anifrolumab: First Approval. Drugs 2021, 81, 1795–1802.
- Heo, Y.-A. Efgartigimod: First Approval. Drugs 2022, 82, 341–348.
- D’Haens, G.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.-F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as Induction Therapy for Crohn’s Disease: Results from the Phase 3 ADVANCE and MOTIVATE Induction Trials. Lancet 2022, 399, 2015–2030.
- Schreiber, S.W.; Ferrante, M.; Panaccione, R.; Colombel, J.F.; Hisamatsu, T.; Lim, A.; Lindsay, J.O.; Rubin, D.T.; Sandborn, W.J.; Neimark, E.; et al. OP26 Risankizumab Induces Early Clinical Remission and Response in Patients with Moderate-to-Severe Crohn’s Disease: Results from the Phase 3 ADVANCE and MOTIVATE Studies. J. Crohns Colitis 2021, 15, S026–S027.
- Dhillon, S. Eptinezumab: First Approval. Drugs 2020, 80, 733–739.
- Lamb, Y.N. Galcanezumab: First Global Approval. Drugs 2018, 78, 1769–1775.
- Hoy, S.M. Fremanezumab: First Global Approval. Drugs 2018, 78, 1829–1834.
- Markham, A. Erenumab: First Global Approval. Drugs 2018, 78, 1157–1161.
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121.
- Marone, G.; Spadaro, G.; Braile, M.; Poto, R.; Criscuolo, G.; Pahima, H.; Loffredo, S.; Levi-Schaffer, F.; Varricchi, G. Tezepelumab: A Novel Biological Therapy for the Treatment of Severe Uncontrolled Asthma. Expert. Opin. Investig. Drugs 2019, 28, 931–940.
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Eng. J. Med. 2021, 384, 1800–1809.
- Keating, G.M. Mepolizumab: First Global Approval. Drugs 2015, 75, 2163–2169.
- Markham, A. Reslizumab: First Global Approval. Drugs 2016, 76, 907–911.
- Markham, A. Benralizumab: First Global Approval. Drugs 2018, 78, 505–511.
- Johnson, T.B.; Cain, J.T.; White, K.A.; Ramirez-Montealegre, D.; Pearce, D.A.; Weimer, J.M. Therapeutic Landscape for Batten Disease: Current Treatments and Future Prospects. Nat. Rev. Neurol. 2019, 15, 161–178.
- Markham, A. Cerliponase Alfa: First Global Approval. Drugs 2017, 77, 1247–1249.
- Karlawish, J.; Grill, J.D. The Approval of Aduhelm Risks Eroding Public Trust in Alzheimer Research and the FDA. Nat. Rev. Neurol. 2021, 17, 523–524.
- Dhillon, S. Aducanumab: First Approval. Drugs 2021, 81, 1437–1443.
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Eng. J. Med. 2021, 384, 1691–1704.
- U.S.A. Food and Drug Administration Novel Drug Approvals for 2021. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 7 September 2022).
- Grilo, A.L.; Mantalaris, A. The Increasingly Human and Profitable Monoclonal Antibody Market. Trends Biotechnol. 2019, 37, 9–16.
- Theocharopoulos, C.; Lialios, P.-P.; Samarkos, M.; Gogas, H.; Ziogas, D.C. Antibody-Drug Conjugates: Functional Principles and Applications in Oncology and Beyond. Vaccines 2021, 9, 1111.
