Precision medicine requires highly sensitive and specific diagnostic strategies with high spatiotemporal resolution. Accurate detection and monitoring of endogenously generated biomarkers at the very early disease stage is of extensive importance for precise diagnosis and treatment. Aggregation-induced emission luminogens (AIEgens) have emerged as a new type of excellent optical agents, which show great promise for numerous biomedical applications. Advances of AIE-based probes for detecting reactive species (including reactive oxygen species (ROS), reactive nitrogen species (RNS), reactive sulfur species (RSS), and reactive carbonyl species (RCS)) and related biomedical applications are introduced. The molecular design strategies for increasing the sensitivity, tuning the response wavelength, and realizing afterglow imaging are summarized, and theranostic applications in reactive species-related major diseases such as cancer, inflammation, and vascular diseases are reviewed.
- aggregation-induced emission
- reactive oxygen nitrogen species
- activatable probe
- theranostics
- fluorescence
- photoacoustic
- afterglow
- bioimaging
1. Introduction
2. Detection of Reactive Oxygen Nitrogen Species
3. Detection of Gasotransmitters
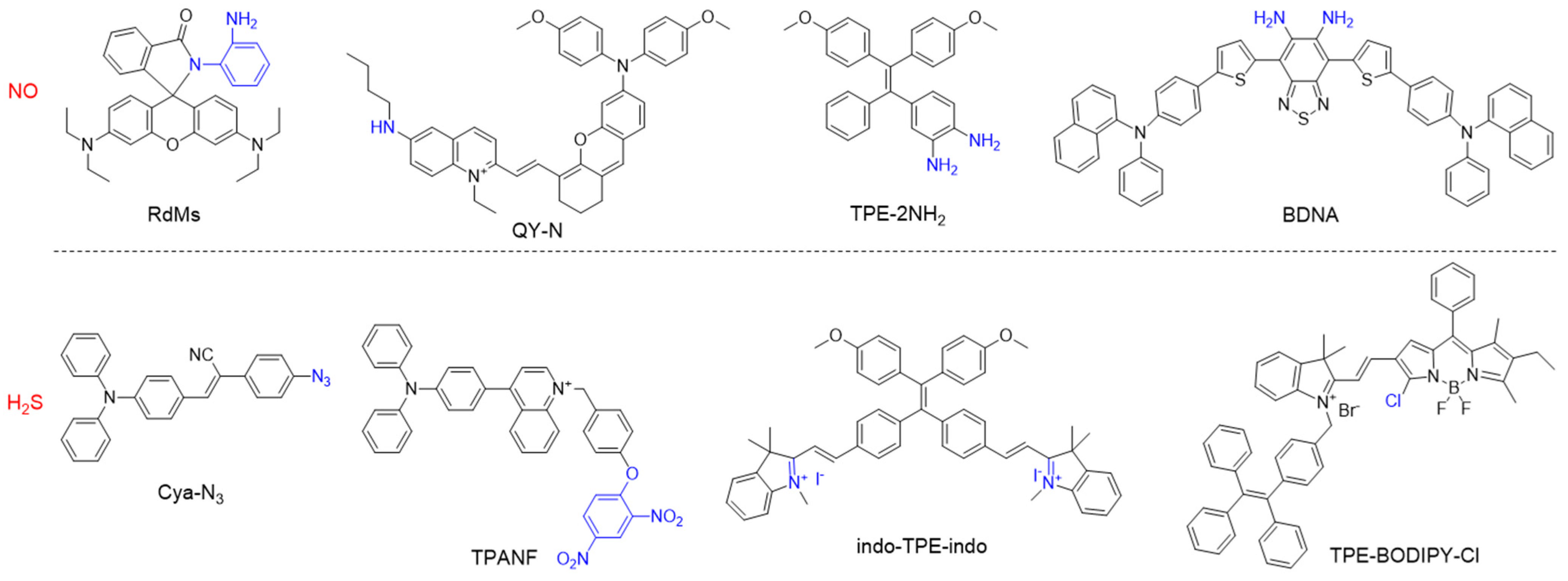
This entry is adapted from the peer-reviewed paper 10.3390/bios12080646
References
- Park, S.M.; Aalipour, A.; Vermesh, O.; Yu, J.H.; Gambhir, S.S. Towards clinically translatable in vivo nanodiagnostics. Nat. Rev. Mater. 2017, 2, 17014.
- Letai, A. Functional precision cancer medicine–moving beyond pure genomics. Nat. Med. 2016, 23, 1028–1035.
- Ashley, E.A. Towards precision medicine. Nat. Rev. Genet. 2016, 17, 507–522.
- Weissleder, R. Scaling down imaging: Molecular mapping of cancer in mice. Nat. Rev. Cancer 2012, 2, 11–18.
- Willmann, J.K.; Bruggen, N.V.; Dinkelborg, L.M.; Gambhir, S.S. Molecular imaging in drug development. Nat. Rev. Drug Discov. 2008, 7, 591–607.
- Nicolson, F.; Kircher, M.F.; Stone, N.; Matousek, P. Spatially offset Raman spectroscopy for biomedical applications. Chem. Soc. Rev. 2021, 50, 556–568.
- Naumova, A.V.; Modo, M.; Moore, A.; Murry, C.E.; Frank, J.A. Clinical imaging in regenerative medicine. Nat. Biotechnol. 2014, 32, 804–818.
- Mustafa, D.A.; Al-Shimmari, H.A.T.; Radhi, M.M. Use of MgCl2 Nanoparticles as Alternative Contrast Media in Magnatic Resonance Imaging Molecular Imaging and Analyzed by Voltammetric Technique. Nano Biomed. Eng. 2020, 12, 148–152.
- Wang, L.V.; Yao, J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638.
- Gottschalk, S.; Degtyaruk, O.; Mc Larney, B.; Rebling, J.; Hutter, M.A.; Deán-Ben, X.L.; Shoham, S.; Razansky, D. Rapid volumetric optoacoustic imaging of neural dynamics across the mouse brain. Nat. Biomed. Eng. 2019, 3, 392–401.
- Li, W.; Yan, Z.; Ren, J.; Qu, X. Manipulating cell fate: Dynamic control of cell behaviors on functional platforms. Chem. Soc. Rev. 2018, 47, 8639–8684.
- Ni, J.-S.; Min, T.; Li, Y.; Zha, M.; Zhang, P.; Ho, L.; Li, K. Planar AIEgens with enhanced solid-state luminescence and ROS generation for multidrug-resistant bacteria treatment. Angew. Chem. Int. Ed. 2020, 59, 10179–10185.
- Li, C.; Chen, G.; Zhang, Y.; Wu, F.; Wang, Q. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 2020, 142, 14789–14804.
- Zhang, Y.; Zhou, J.; Peng, S.; Yu, W.; Fan, X.; Liu, W.; Ye, Z.; Qi, J.; Feng, Z.; Qian, J. Hot-Band-Absorption-Induced Anti-Stokes Fluorescence of Aggregation-Induced Emission Dots and the Influence on the Nonlinear Optical Effect. Biosensors 2021, 11, 468.
- Liu, R.; Xu, Y.; Xu, K.; Dai, Z. Current trends and key considerations in the clinical translation of targeted fluorescent probes for intraoperative navigation. Aggregate 2021, 2, e23.
- Zhou, J.; del Rosal, B.; Jaque, D.; Uchiyama, S.; Jin, D. Advances and challenges for fluorescence nanothermometry. Nat. Methods 2020, 17, 967–980.
- Fan, Y.; Wang, P.; Lu, Y.; Wang, R.; Zhou, L.; Zheng, X.; Li, X.; Piper, J.A.; Zhang, F. Lifetime-engineered NIR-II nanoparticles unlock multiplexed in vivo imaging. Nat. Nanotechnol. 2018, 13, 941–946.
- Wu, Y.; Zeng, F.; Zhao, Y.; Wu, S. Emerging contrast agents for multispectral optoacoustic imaging and their biomedical applications. Chem. Soc. Rev. 2021, 50, 7924–7940.
- Ji, X.; Ge, L.; Liu, C.; Tang, Z.; Xiao, Y.; Chen, W.; Lei, Z.; Gao, W.; Blake, S.; De, D.; et al. Capturing functional two-dimensional nanosheets from sandwich-structure vermiculite for cancer theranostics. Nat. Commun. 2021, 12, 1124.
- Kang, M.; Zhang, Z.; Song, N.; Li, M.; Sun, P.; Chen, X.; Wang, D.; Tang, B.Z. Aggregation-enhanced theranostics: AIE sparkles in biomedical field. Aggregate 2020, 1, 80–106.
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: From strategic design toward molecular imaging and theranostics. Chem. Rev. 2022, 122, 209–268.
- Qi, J.; Fang, Y.; Kwok, R.T.K.; Zhang, X.; Hu, X.; Lam, J.W.Y.; Ding, D.; Tang, B.Z. Highly Stable Organic Small Molecular Nanoparticles as an Advanced and Biocompatible Phototheranostic Agent of Tumor in Living Mice. ACS Nano 2017, 11, 7177–7188.
- Cai, Y.; Chen, X.; Si, J.; Mou, X.; Dong, X. All-in-One Nanomedicine: Multifunctional Single-Component Nanoparticles for Cancer Theranostics. Small 2021, 17, 2103072.
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108.
- Wu, L.; Huang, J.; Pu, K.; James, T.D. Dual-locked spectroscopic probes for sensing and therapy. Nat. Rev. Chem. 2021, 5, 406–421.
- Qi, J.; Feng, L.; Zhang, X.; Zhang, H.; Huang, L.; Zhou, Y.; Zhao, Z.; Duan, X.; Xu, F.; Kwok, R.T.K.; et al. Facilitation of molecular motion to develop turn-on photoacoustic bioprobe for detecting nitric oxide in encephalitis. Nat. Commun. 2021, 12, 960.
- Antaris, A.L.; Chen, H.; Cheng, K.; Sun, Y.; Hong, G.; Qu, C.; Diao, S.; Deng, Z.; Hu, X.; Zhang, B.; et al. A small-molecule dye for NIR-II imaging. Nat. Mater. 2016, 15, 235–242.
- Yang, J.; Zhang, Y.; Wu, X.; Dai, W.; Chen, D.; Shi, J.; Tong, B.; Peng, Q.; Xie, H.; Cai, Z.; et al. Rational design of pyrrole derivatives with aggregation-induced phosphorescence characteristics for time-resolved and two-photon luminescence imaging. Nat. Commun. 2021, 12, 4883.
- Ding, Z.; Gu, Y.; Zheng, C.; Gu, Y.; Yang, J.; Li, D.; Xu, Y.; Wang, P. Organic small molecule-based photothermal agents for cancer therapy: Design strategies from single-molecule optimization to synergistic enhancement. Coordin. Chem. Rev. 2022, 464, 214564.
- Ji, C.; Cheng, W.; Yuan, Q.; Müllen, K.; Yin, M. From Dyestuff Chemistry to Cancer Theranostics: The Rise of Rylenecarboximides. Acc. Chem. Res. 2019, 52, 2266–2277.
- Yang, J.; Fang, M.; Li, Z. Organic luminescent materials: The concentration on aggregates from aggregation-induced emission. Aggregate 2020, 1, 6–18.
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1, 2, 3, 4, 5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741.
- Chen, Y.; Lam, J.W.Y.; Kwok, R.T.K.; Liu, B.; Tang, B.Z. Aggregation-induced emission: Fundamental understanding and future developments. Mater. Horiz. 2019, 6, 428–433.
- Gao, M.; Tang, B.Z. AIE-based cancer theranostics. Coordin. Chem. Rev. 2020, 402, 213076.
- Zha, M.; Yang, G.; Li, Y.; Zhang, C.; Li, B.; Li, K. Recent Advances in AIEgen-Based Photodynamic Therapy and Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2101066.
- Zang, T.; Xie, Y.; Su, S.; Liu, F.; Chen, Q.; Jing, J.; Zhang, R.; Niu, G.; Zhang, X. In Vitro Light-Up Visualization of a Subunit-Specific Enzyme by an AIE Probe via Restriction of Single Molecular Motion. Angew. Chem. Int. Ed. 2020, 59, 10003–10007.
- Zhao, Z.; Zhang, H.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: New vistas at the aggregate level. Angew. Chem. Int. Ed. 2020, 59, 9888–9907.
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388.
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479.
- Feng, G.; Liu, B. Aggregation-Induced Emission (AIE) Dots: Emerging Theranostic Nanolights. Acc. Chem. Res. 2018, 51, 1404–1414.
- Wang, Z.; Zhou, Y.; Xu, R.; Xu, Y.; Dang, D.; Shen, Q.; Meng, L.; Tang, B.Z. Seeing the unseen: AIE luminogens for super-resolution imaging. Coordin. Chem. Rev. 2022, 451, 214279.
- Yang, G.; Ni, J.-S.; Li, Y.; Zha, M.; Tu, Y.; Li, K. Acceptor Engineering for Optimized ROS Generation Facilitates Reprogramming Macrophages to M1 Phenotype in Photodynamic Immunotherapy. Angew. Chem. Int. Ed. 2021, 60, 5386–5393.
- Qi, J.; Sun, C.; Li, D.; Zhang, H.; Yu, W.; Zebibula, A.; Lam, J.W.Y.; Xi, W.; Zhu, L.; Cai, F.; et al. Aggregation-Induced Emission Luminogen with Near-Infrared-II Excitation and Near-Infrared-I Emission for Ultradeep Intravital Two-Photon Microscopy. ACS Nano 2018, 12, 7936–7945.
- Li, Q.; Li, Y.; Min, T.; Gong, J.; Du, L.; Phillips, D.L.; Liu, J.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; et al. Time-Dependent Photodynamic Therapy for Multiple Targets: A Highly Efficient AIE-Active Photosensitizer for Selective Bacterial Elimination and Cancer Cell Ablation. Angew. Chem. Int. Ed. 2020, 59, 9470–9477.
- He, Z.; Gao, Y.; Zhang, H.; Xue, Y.; Meng, F.; Luo, L. Mitochondrion-Anchored Photosensitizer with Near Infrared-I Aggregation-Induced Emission for Near Infrared-II Two-Photon Photodynamic Therapy. Adv. Healthc. Mater. 2021, 10, 2101056.
- Naghibi, S.; Chen, T.; Ghahfarokhi, A.J.; Tang, Y. AIEgen-enhanced protein imaging: Probe design and sensing mechanisms. Aggregate 2021, 2, e41.
- Huang, J.; Nie, H.; Zeng, J.; Zhuang, Z.; Gan, S.; Cai, Y.; Guo, J.; Su, S.-J.; Zhao, Z.; Tang, B.Z. Highly Efficient Nondoped OLEDs with Negligible Efficiency Roll-Off Fabricated from Aggregation-Induced Delayed Fluorescence Luminogens. Angew. Chem. Int. Ed. 2017, 56, 12971–12976.
- Xu, Y.; Xu, R.; Wang, Z.; Zhou, Y.; Shen, Q.; Ji, W.; Dang, D.; Meng, L.; Tang, B.Z. Recent advances in luminescent materials for super-resolution imaging via stimulated emission depletion nanoscopy. Chem. Soc. Rev. 2021, 50, 667–690.
- Liu, C.; Wang, X.; Liu, J.; Yue, Q.; Chen, S.; Lam, J.W.Y.; Luo, L.; Tang, B.Z. Near-Infrared AIE Dots with Chemiluminescence for Deep-Tissue Imaging. Adv. Mater. 2020, 32, 2004685.
- Hu, Q.; Hu, H.; Zhang, X.; Fan, K.; Hong, Y.; Raston, C.L.; Tang, Y. In situ monitored vortex fluidic-mediated protein refolding/unfolding using an aggregation-induced emission bioprobe. Molecules 2021, 26, 4273.
- Cai, X.; Liu, B. Aggregation-Induced Emission: Recent Advances in Materials and Biomedical Applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886.
- Qi, J.; Ou, H.; Liu, Q.; Ding, D. Gathering brings strength: How organic aggregates boost disease phototheranostics. Aggregate 2021, 2, 95–113.
- Zhou, T.; Hu, R.; Wang, L.; Qiu, Y.; Zhang, G.; Deng, Q.; Zhang, H.; Yin, P.; Situ, B.; Zhan, C.; et al. An AIE-Active Conjugated Polymer with High ROS-Generation Ability and Biocompatibility for Efficient Photodynamic Therapy of Bacterial Infections. Angew. Chem. Int. Ed. 2020, 59, 9952–9956.
- Zhang, Z.; Fang, X.; Liu, Z.; Liu, H.; Chen, D.; He, S.; Zheng, J.; Yang, B.; Qin, W.; Zhang, X.; et al. Semiconducting Polymer Dots with Dual-Enhanced NIR-IIa Fluorescence for Through-Skull Mouse-Brain Imaging. Angew. Chem. Int. Ed. 2020, 59, 3691–3698.
- Liu, M.; Chen, Y.; Guo, Y.; Yuan, H.; Cui, T.; Yao, S.; Jin, S.; Fan, H.; Wang, C.; Xie, R.; et al. Golgi apparatus-targeted aggregation-induced emission luminogens for effective cancer photodynamic therapy. Nat. Commun. 2022, 13, 2179.
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40.
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462.
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C.S. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193.
- Wang, Z.; Zhang, Y.; Ju, E.; Liu, Z.; Cao, F.; Chen, Z.; Ren, J.; Qu, X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018, 9, 3334.
- Tang, D.; Wang, Y.; Wijaya, A.; Liu, B.; Maruf, A.; Wang, J.; Xu, J.; Liao, X.; Wu, W.; Wang, G. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen. Biomater. 2021, 8, rbab033.
- Zhang, Y.; Yang, H.; Wei, D.; Zhang, X.; Wang, J.; Wu, X.; Chang, J. Mitochondria-targeted nanoparticles in treatment of neurodegenerative diseases. Exploration 2021, 1, 20210115.
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.-S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30.
- Nguyen, K.T.; Zhao, Y. Engineered Hybrid Nanoparticles for On-Demand Diagnostics and Therapeutics. Acc. Chem. Res. 2015, 48, 3016–3025.
- Wang, C.; Ding, S.; Wang, S.; Shi, Z.; Pandey, N.K.; Chudal, L.; Wang, L.; Zhang, Z.; Wen, Y.; Yao, H.; et al. Endogenous tumor microenvironment-responsive multifunctional nanoplatforms for precision cancer theranostics. Coordin. Chem. Rev. 2021, 426, 213529.
- Barani, M.; Mukhtar, M.; Rahdar, A.; Sargaz, S.; Pandey, S.; Kang, M. Recent Advances in Nanotechnology-Based Diagnosis and Treatments of Human Osteosarcoma. Biosensors 2021, 11, 55.
- Paul, B.D.; Snyder, S.H. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Bio. 2012, 13, 499–507.
- Jiang, Y.; Pu, K. Molecular Probes for Autofluorescence-Free Optical Imaging. Chem. Rev. 2021, 121, 13086–13131.
- Yang, X.; Zhang, D.; Ye, Y.; Zhao, Y. Recent advances in multifunctional fluorescent probes for viscosity and analytes. Coordin. Chem. Rev. 2022, 453, 214336.
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555.
- Zhan, Z.; Dai, Y.; Li, Q.; Lv, Y. Small molecule-based bioluminescence and chemiluminescence probes for sensing and imaging of reactive species. TrAC Trend. Anal. Chem. 2021, 134, 116129.
- Li, J.; Wang, T.; Jiang, F.; Hong, Z.; Su, X.; Li, S.; Han, S. Activatable Dual ROS-Producing Probe for Dual Organelle-Engaged Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 4618–4628.
- Hu, J.-J.; Jiang, W.; Yuan, L.; Duan, C.; Yuan, Q.; Long, Z.; Lou, X.; Xia, F. Recent advances in stimuli-responsive theranostic systems with aggregation-induced emission characteristics. Aggregate 2021, 2, 48–65.
- Tang, G.; He, J.; Liu, J.; Yan, X.; Fan, K. Nanozyme for tumor therapy: Surface modification matters. Exploration 2021, 1, 75–89.
- Reza, A.H.M.M.; Zhu, X.; Qin, J.; Tang, Y. Microalgae-Derived Health Supplements to Therapeutic Shifts: Redox-Based Study Opportunities with AIE-Based Technologies. Adv. Healthc. Mater. 2021, 10, 2101223.
- Mujika, J.I.; Uranga, J.; Matxain, J.M. Computational Study on the Attack of •OH Radicals on Aromatic Amino Acids. Chem.-Eur. J. 2013, 19, 6862–6873.
- Vicente-Gutierrez, C.; Bonora, N.; Bobo-Jimenez, V.; Jimenez-Blasco, D.; Lopez-Fabuel, I.; Fernandez, E.; Josephine, C.; Bonvento, G.; Enriquez, J.A.; Almeida, A.; et al. Astrocytic mitochondrial ROS modulate brain metabolism and mouse behaviour. Nat. Metab. 2019, 1, 201–211.
- Vaccaro, A.; Dor, Y.K.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulj, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.
- Yardeni, T.; Tanes, C.E.; Bittinger, K.; Mattei, L.M.; Schaefer, P.M.; Singh, L.N.; Wu, G.D.; Murdock, D.G.; Wallace, D.C. Host mitochondria influence gut microbiome diversity: A role for ROS. Sci. Signal. 2019, 12, eaaw3159.
- Cheng, D.; Xu, W.; Gong, X.; Yuan, L.; Zhang, X.-B. Design Strategy of Fluorescent Probes for Live Drug-Induced Acute Liver Injury Imaging. Acc. Chem. Res. 2021, 54, 403–415.
- Li, C.; Li, S.; Zhao, J.; Sun, M.; Wang, W.; Lu, M.; Qu, A.; Hao, C.; Chen, C.; Xu, C.; et al. Ultrasmall Magneto-chiral Cobalt Hydroxide Nanoparticles Enable Dynamic Detection of Reactive Oxygen Species in Vivo. J. Am. Chem. Soc. 2022, 144, 1580–1588.
- Zhang, X.; Chen, Y.; He, H.; Wang, S.; Lei, Z.; Zhang, F. ROS/RNS and Base Dual Activatable Merocyanine-Based NIR-II Fluorescent Molecular Probe for in vivo Biosensing. Angew. Chem. Int. Ed. 2021, 60, 26337–26341.
- Li, J.; Rao, J.; Pu, K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials 2018, 155, 217–235.
- Wang, H.; Wang, X.; Li, P.; Dong, M.; Yao, S.Q.; Tang, B. Fluorescent probes for visualizing ROS-associated proteins in disease. Chem. Sci. 2021, 12, 11620–11646.
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent advances in polymeric nanoparticles for enhanced fluorescence and photoacoustic imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809.
- Wang, D.; Tang, B.Z. Aggregation-induced emission luminogens for activity-based sensing. Acc. Chem. Res. 2019, 52, 2559–2570.
- Niu, G.; Zhang, R.; Shi, X.; Park, H.; Xie, S.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE luminogens as fluorescent bioprobes. TrAC Trend. Anal. Chem. 2020, 123, 115769.
- Li, H.; Kim, H.; Han, J.; Nguyen, V.-N.; Peng, X.; Yoon, J. Activity-based smart AIEgens for detection, bioimaging, and therapeutics: Recent progress and outlook. Aggregate 2021, 2, e51.
- Ouyang, J.; Sun, L.; Zeng, F.; Wu, S. Biomarker-activatable probes based on smart AIEgens for fluorescence and optoacoustic imaging. Coordin. Chem. Rev. 2022, 458, 214438.
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940.
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes based on AIE fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453.
- Qian, J.; Tang, B.Z. AIE luminogens for bioimaging and theranostics: From organelles to animals. Chem 2017, 3, 56–91.
- Qi, J.; Chen, C.; Ding, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens: Union Is Strength, Gathering Illuminates Healthcare. Adv. Healthc. Mater. 2018, 7, 1800477.
- Zhang, H.; Zhao, Z.; Turley, A.T.; Wang, L.; McGonigal, P.R.; Tu, Y.; Li, Y.; Wang, Z.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Aggregate science: From structures to properties. Adv. Mater. 2020, 32, 2001457.
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172.
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984.
- Nguyen, V.-N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coordin. Chem. Rev. 2021, 439, 213936.
- Jiang, G.; Li, C.; Liu, X.; Chen, Q.; Li, X.; Gu, X.; Zhang, P.; Lai, Q.; Wang, J. Lipid droplet-targetable fluorescence guided photodynamic therapy of cancer cells with an activatable AIE-active fluorescent probe for hydrogen peroxide. Adv. Optical Mater. 2020, 8, 2001119.
- Miller, E.W.; Tulyathan, O.; Isacoff, E.Y.; Chang, C.J. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 2007, 3, 349.
- Gao, X.; Feng, G.; Manghnani, P.N.; Hu, F.; Jiang, N.; Liu, J.; Liu, B.; Sun, J.Z.; Tang, B.Z. A two-channel responsive fluorescent probe with AIE characteristics and its application for selective imaging of superoxide anions in living cells. Chem. Commun. 2017, 53, 1653–1656.
- Xiao, H.; Zhang, W.; Li, P.; Zhang, W.; Wang, X.; Tang, B. Versatile Fluorescent Probes for Imaging the Superoxide Anion in Living Cells and In Vivo. Angew. Chem. Int. Ed. 2020, 59, 4216–4230.
- Duan, Q.; Zheng, G.; Li, Z.; Cheng, K.; Zhang, J.; Yang, L.; Jiang, Y.; Zhang, H.; He, J.; Sun, H. An ultra-sensitive ratiometric fluorescent probe for hypochlorous acid detection by the synergistic effect of AIE and TBET and its application of detecting exogenous/endogenous HOCl in living cells. J. Mater. Chem. B 2019, 7, 5125–5131.
- Qiao, W.; Ma, T.; Wang, S.; Li, L.; Liu, M.; Jiang, H.; Wu, Y.; Zhu, J.; Li, Z. Designing Squaraine Dyes with Bright Deep-Red Aggregation-Induced Emission for Specific and Ratiometric Fluorescent Detection of Hypochlorite. Adv. Funct. Mater. 2021, 31, 2105452.
- Wu, W.; Mao, D.; Cai, X.; Duan, Y.; Hu, F.; Kong, D.; Liu, B. ONOO− and ClO− Responsive Organic Nanoparticles for Specific in Vivo Image-Guided Photodynamic Bacterial Ablation. Chem. Mater. 2018, 30, 3867–3873.
- Jiang, G.; Li, C.; Lai, Q.; Liu, X.; Chen, Q.; Zhang, P.; Wang, J.; Tang, B.Z. An easily available ratiometric AIE probe for peroxynitrite in vitro and in vivo imaging. Sensor. Actuat. B Chem. 2021, 329, 129223.
- Han, X.; Yang, X.; Zhang, Y.; Li, Z.; Cao, W.; Zhang, D.; Ye, Y. A novel activatable AIEgen fluorescent probe for peroxynitrite detection and its application in EC1 cells. Sensor. Actuat. B Chem. 2020, 321, 128510.
- Zeng, Z.; Liew, S.S.; Wei, X.; Pu, K. Hemicyanine-Based Near-Infrared Activatable Probes for Imaging and Diagnosis of Diseases. Angew. Chem. Int. Ed. 2021, 60, 26454–26475.
- Vassalle, C.; Maltinti, M.; Sabatino, L. Targeting oxidative stress for disease prevention and therapy: Where do we stand, and where do we go from here. Molecules 2020, 25, 2653.
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. ROS Responsive Nanoplatform with Two-Photon AIE Imaging for Atherosclerosis Diagnosis and “Two-Pronged” Therapy. Small 2020, 16, 2003253.
- Zhang, W.; Liu, W.; Li, P.; Huang, F.; Wang, H.; Tang, B. Rapid-Response Fluorescent Probe for Hydrogen Peroxide in Living Cells Based on Increased Polarity of C−B Bonds. Anal. Chem. 2015, 87, 9825–9828.
- Li, W.; Wang, L.; Tang, H.; Cao, D. An interface-targeting and H2O2-activatable probe liberating AIEgen: Enabling on-site imaging and dynamic movement tracking of lipid droplets. Chem. Commun. 2019, 55, 4491–4494.
- Mao, D.; Wu, W.; Ji, S.; Chen, C.; Hu, F.; Kong, D.; Ding, D.; Liu, B. Chemiluminescence-Guided Cancer Therapy Using a Chemiexcited Photosensitizer. Chem 2017, 3, 991–1007.
- Xu, L.; Sun, L.; Zeng, F.; Wu, S. Near-Infrared Fluorescent Nanoprobe for Detecting Hydrogen Peroxide in Inflammation and Ischemic Kidney Injury. Chin. J. Chem. 2020, 38, 1304–1310.
- Cheng, Y.; Dai, J.; Sun, C.; Liu, R.; Zhai, T.; Lou, X.; Xia, F. An Intracellular H2O2-Responsive AIEgen for the Peroxidase-Mediated Selective Imaging and Inhibition of Inflammatory Cells. Angew. Chem. Int. Ed. 2018, 57, 3123–3127.
- Ma, B.; Xu, H.; Zhuang, W.; Wang, Y.; Li, G.; Wang, Y. Reactive Oxygen Species Responsive Theranostic Nanoplatform for Two-Photon Aggregation-Induced Emission Imaging and Therapy of Acute and Chronic Inflammation. ACS Nano 2020, 14, 5862–5873.
- Shatalin, K.; Shatalina, E.; Mironov, A.; Nudler, E. H2S: A Universal Defense Against Antibiotics in Bacteria. Science 2011, 334, 986–990.
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203.
- Kumar, N.; Bhalla, V.; Kumar, M. Recent developments of fluorescent probes for the detection of gasotransmitters (NO, CO and H2S). Coordin. Chem. Rev. 2013, 257, 2335–2347.
- Opoku-Damoah, Y.; Zhang, R.; Ta, H.T.; Xu, Z.P. Therapeutic gas-releasing nanomedicines with controlled release: Advances and perspectives. Exploration 2022, 2, 20210181.
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015, 14, 623–641.
- Zhou, L.; Li, X.; Wang, K.; Shen, F.; Zhang, L.; Li, P.; Shang, T.; Wang, J.; Huang, N. Cu∥-loaded polydopamine coatings with in situ nitric oxide generation function for improved hemocompatibility. Regen. Biomater. 2020, 7, 153–160.
- Qian, Y.; Matson, J.B. Gasotransmitter delivery via self-assembling peptides: Treating diseases with natural signaling gases. Adv. Drug Delivery Rev. 2017, 110–111, 137–156.
- Li, Z.; Polhemus, D.J.; Lefer, D.J. Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease. Circ. Res. 2018, 123, 590–600.
- Zhou, Y.; Zhang, X.; Yang, S.; Li, Y.; Qing, Z.; Zheng, J.; Li, J.; Yang, R. Ratiometric Visualization of NO/H2S Cross-Talk in Living Cells and Tissues Using a Nitroxyl-Responsive Two-Photon Fluorescence Probe. Anal. Chem. 2017, 89, 4587–4594.
- Yang, M.; Fan, J.; Du, J.; Peng, X. Small-molecule fluorescent probes for imaging gaseous signaling molecules: Current progress and future implications. Chem. Sci. 2020, 11, 5127–5141.
- Zhu, T.; Ren, N.; Liu, X.; Dong, Y.; Wang, R.; Gao, J.; Sun, J.; Zhu, Y.; Wang, L.; Fan, C.; et al. Probing the Intracellular Dynamics of Nitric Oxide and Hydrogen Sulfide Using an Activatable NIR II Fluorescence Reporter. Angew. Chem. Int. Ed. 2021, 60, 8450–8454.
- Liu, L.; Zhang, F.; Xu, B.; Tian, W. Silica nanoparticles based on an AIE-active molecule for ratiometric detection of RNS in vitro. J. Mater. Chem. B 2017, 5, 9197–9203.
- Hu, W.; Xie, M.; Zhao, H.; Tang, Y.; Yao, S.; He, T.; Ye, C.; Wang, Q.; Lu, X.; Huang, W.; et al. Nitric oxide activatable photosensitizer accompanying extremely elevated two-photon absorption for efficient fluorescence imaging and photodynamic therapy. Chem. Sci. 2018, 9, 999–1005.
- Lucero, M.Y.; East, A.K.; Reinhardt, C.J.; Sedgwick, A.C.; Su, S.; Lee, M.C.; Chan, J. Development of NIR-II photoacoustic probes tailored for deep-tissue sensing of nitric oxide. J. Am. Chem. Soc. 2021, 143, 7196–7202.
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618.
- Zhou, Y.; Mazur, F.; Fan, Q.; Chandrawati, R. Synthetic nanoprobes for biological hydrogen sulfide detection and imaging. View 2022, 3, 20210008.
- Sun, L.; Ouyang, J.; Ma, Y.; Zeng, Z.; Zeng, C.; Zeng, F.; Wu, S. An Activatable Probe with Aggregation-Induced Emission for Detecting and Imaging Herbal Medicine Induced Liver Injury with Optoacoustic Imaging and NIR-II Fluorescence Imaging. Adv. Healthc. Mater. 2021, 10, 2100867.
