Neuropeptides are mainly secreted from the human central and peripheral nervous systems. Neuropeptides bind to its cognate rhodopsin-like G-protein coupled receptor (GPCR) and perform various physiological functions. Conventional cancer treatments in clinical practice still present many drawbacks due to the lack of selectivity toward the target cell, drug-resistance, and side-effects, thus pushing for the development of new therapeutic agents and therapies. Recent research suggests that neuropeptides influence cancer cell proliferation, invasion, metastasis, and angiogenesis and, therefore, they could be exploited as a target for novel anticancer therapies. Very recently, targeted approaches that inhibit neuropeptides and their associated receptors are being developed in cancer treatment.
- neuropeptide
- receptor
- inhibitors
- colorectal cancer
- bombesin
- neurotensin
- vasoactive intestinal peptide
- substance P
- neuropeptide Y
- orexins
1. Neuropeptides
| Neuropeptides | Number of Amino Acids |
Discovery | Related Cancers | Tumorigenic Properties | Refs. |
|---|---|---|---|---|---|
| Neurotensin | 13 | Carraway and Leeman in 1973 | Pancreatic, lung, breast, prostate, and colorectal cancer | Increased cell proliferation and migration | [3][4] |
| Vasoactive intestinal polypeptide (VIP) | 28 | Said and Mutt in 1970 | Neuroblastoma, pituitary adenomas, colorectal cancer, endometrial, and lung cancer | Increased cell proliferation, metastasis, invasion, and angiogenesis | [5][6] |
| Substance P | 11 | Von Euler and Gaddum in 1931 | Glioblastoma, breast, acute lymphoblastic Leukemia, colorectal cancer, melanoma, and gastric cancer |
Increased cell proliferation, migration, invasion, and angiogenesis; pro-inflammatory effect | [7][8] |
| Bombesin | 14 | Battey and Wada in 1991 | Prostate, gastric, lung, breast, colorectal cancer, renal cell carcinoma, small cell lung carcinoma, neuroendocrine, squamous, colon, and pituitary cancer |
Promoted vascularization, tumor growth, and differentiation | [9][10][11][12] |
| Neuropeptide Y | 36 | Tatemoto and Mutt in 1982 | Neuroblastoma, colorectal cancer, breast, Ewing sarcoma, and prostate cancer | Induced cell growth, vascularization, and angiogenesis; pro-inflammatory effect | [13][14][15] |
| Calcitonin gene-related peptide (CGRP) | 37 | Amara and colleagues in 1982 | Prostate, lung, colorectal cancer, pancreatic, ovarian, endometrial, pituitary, renal, and hepatic cancer | Promoted angiogenesis, lymphangiogenesis, cell growth, neovascularization, proliferation, and migration | [16][17][18][19] |
2. Emerging Neuropeptides Inhibitors in Colorectal Cancer
2.1. Bombesin
2.2. Neurotensin
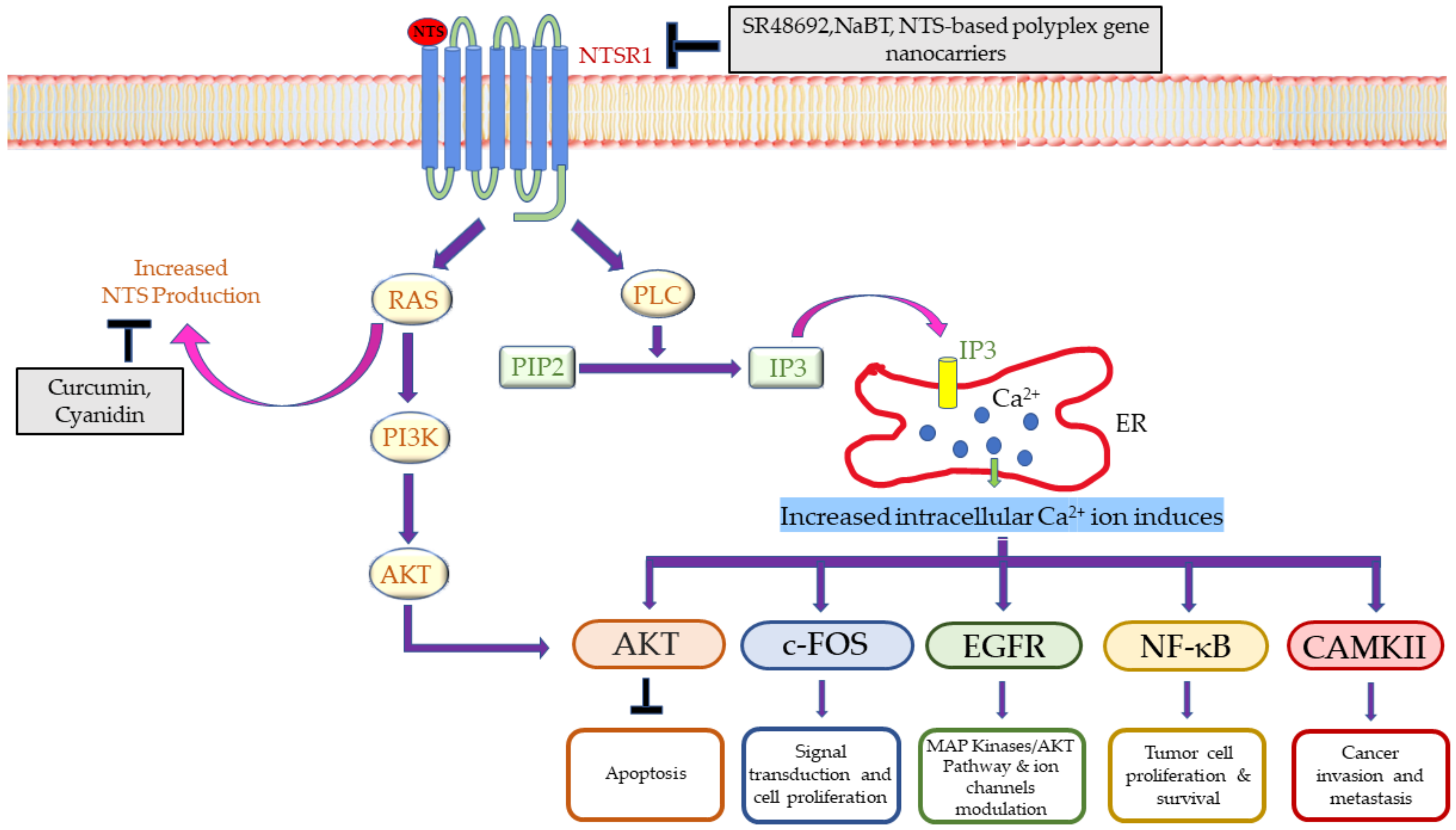
2.3. Vasoactive Intestinal Peptide
2.4. Substance P
2.5. Neuropeptide Y
2.6. Orexins
This entry is adapted from the peer-reviewed paper 10.3390/app12188990
References
- Schuller, H.M. Neurotransmission and cancer: Implications for prevention and therapy. Anti-Cancer Drugs 2008, 19, 655–671.
- Itoh, N.; Semba, S.; Ito, M.; Takeda, H.; Kawata, S.; Yamakawa, M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer 2002, 94, 3127–3134.
- Dupouy, S.; Mourra, N.; Gompel, A.; Alifano, M.; Forgez, P. The potential use of the neurotensin high affinity receptor 1 as a biomarker for cancer progression and as a component of personalized medicine in selective cancers. Biochimie 2011, 93, 1369–1378.
- Qiu, S.; Pellino, G.; Fiorentino, F.; Rasheed, S.; Darzi, A.; Tekkis, P.; Kontovounisios, C. A Review of the Role of Neurotensin and Its Receptors in Colorectal Cancer. Gastroenterol. Res. Pract. 2017, 2017, 6456257.
- Tang, B.; Yong, X.; Xie, R.; Li, Q.-W.; Yang, S.-M. Vasoactive intestinal peptide receptor-based imaging and treatment of tumors (Review). Int. J. Oncol. 2014, 44, 1023–1031.
- Zawilska, J.B.; Niewiadomski, P.; Nowak, J.Z. Receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in the goose cerebral cortex. Pol. J. Pharmacol. 2004, 56, 203–211.
- Garnier, A.; Vykoukal, J.; Hubertus, J.; Alt, E.; Von Schweinitz, D.; Kappler, R.; Berger, M.; Ilmer, M. Targeting the neurokinin-1 receptor inhibits growth of human colon cancer cells. Int. J. Oncol. 2015, 47, 151–160.
- Mehboob, R.; Gilani, S.A.; Hassan, A.; Tanvir, I.; Javaid, S.; Khalid, S.; Hasan, S.; Waseem, H.; Alwazzan, A.; Munoz, M. Prognostic Significance of Substance P/Neurokinin 1 Receptor and Its Association with Hormonal Receptors in Breast Carcinoma. Biomed. Res. Int. 2020, 2021, 5577820.
- Bajo, A.M.; Schally, A.V.; Groot, K.; Szepeshazi, K. Bombesin antagonists inhibit proangiogenic factors in human experimental breast cancers. Br. J. Cancer 2004, 90, 245–252.
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765.
- Moreno, P.; Ramos-Álvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073.
- Moody, T.W.; Lee, L.; Ramos-Alvarez, I.; Iordanskaia, T.; Mantey, S.A.; Jensen, R.T. Bombesin Receptor Family Activation and CNS/Neural Tumors: Review of Evidence Supporting Possible Role for Novel Targeted Therapy. Front. Endocrinol. 2021, 12, 728088.
- Li, J.; Tian, Y.; Wu, A. Neuropeptide Y receptors: A promising target for cancer imaging and therapy. Regen. Biomater. 2015, 2, 215–219.
- Tilan, J.; Kitlinska, J. Neuropeptide Y (NPY) in tumor growth and progression: Lessons learned from pediatric oncology. Neuropeptides 2016, 55, 55–66.
- Galli, S.; Naranjo, A.; Van Ryn, C.; Tilan, J.U.; Trinh, E.; Yang, C.; Tsuei, J.; Hong, S.-H.; Wang, H.; Izycka-Swieszewska, E.; et al. Neuropeptide Y as a Biomarker and Therapeutic Target for Neuroblastoma. Am. J. Pathol. 2016, 186, 3040–3053.
- Martinez, A.; Miller, M.J.; Unsworth, E.J.; Siegfried, J.M.; Cuttitta, F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology 1995, 136, 4099–4105.
- Rocchi, P.; Boudouresque, F.; Zamora, A.J.; Muracciole, X.; Lechevallier, E.; Martin, P.-M.; Ouafik, L.H. Expression of adrenomedullin and peptide amidation activity in human prostate cancer and in human prostate cancer cell lines. Cancer Res. 2001, 61, 1196–1206.
- Michelsen, J.; Thiesson, H.; Walter, S.; Ottosen, P.D.; Skøtt, O.; Jensen, B.L. Tissue expression and plasma levels of adrenomedullin in renal cancer patients. Clin. Sci. 2006, 111, 61–70.
- Nakata, T.; Seki, N.; Miwa, S.; Kobayashi, A.; Soeda, J.; Nimura, Y.; Kawasaki, S.; Miyagawa, S. Identification of genes associated with multiple nodules in hepatocellular carcinoma using cDNA microarray: Multicentric occurrence or intrahepatic metastasis? Hepatogastroenterology 2008, 55, 865–872.
- Tsang, W.Y.; Chan, J.K. Neural invasion in intraductal carcinoma of the breast. Hum. Pathol. 1992, 23, 202–204.
- Wang, J.; He, Y. Diagnostic role of NPY methylation in patients with colorectal cancer. JUSTC 2022, 52, 2.
- Souazé, F.; Viardot-Foucault, V.; Roullet, N.; Toy-Miou-Leong, M.; Gompel, A.; Bruyneel, E.; Comperat, E.; Faux, M.C.; Mareel, M.; Rostène, W.; et al. Neurotensin receptor 1 gene activation by the Tcf/β-catenin pathway is an early event in human colonic adenomas. Carcinogenesis 2006, 27, 708–716.
- Dupouy, S.; Viardot-Foucault, V.; Alifano, M.; Souazé, F.; Plu-Bureau, G.; Chaouat, M.; Lavaur, A.; Hugol, D.; Gespach, C.; Gompel, A.; et al. The neurotensin receptor-1 pathway contributes to human ductal breast cancer progression. PLoS ONE 2009, 4, e4223.
- Munoz, M.; Covenas, R. Involvement of substance P and the NK-1 receptor in cancer progression. Peptides 2013, 48, 1–9.
- Muñoz, M.; Coveñas, R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids 2014, 46, 1727–1750.
- Muñoz, M.; Coveñas, R. Neurokinin-1 receptor antagonists as antitumor drugs in gastrointestinal cancer: A new approach. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2016, 22, 260.
- Maoret, J.J.; Anini, Y.; Rouyer-Fessard, C.; Gully, D.; Laburthe, M. Neurotensin and a non-peptide neurotensin receptor antagonist control human colon cancer cell growth in cell culture and in cells xenografted into nude mice. Int. J. Cancer 1999, 80, 448–454.
- Brzozowska, M.; Całka, J. Occurrence and distribution of galanin in the physiological and inflammatory states in the mammalian gastrointestinal tract. Front. Immunol. 2021, 11, 602070.
- Zhang, L.; Fang, P.; Chai, C.; Shao, L.; Mao, H.; Qiao, D.; Kong, G.; Dong, X.; Shi, M.; Zhang, Z. Galanin expression is down-regulated in patients with gastric cancer. J. Int. Med. Res. 2019, 47, 1241–1249.
- Kwiatkowski, P.; Godlewski, J.; Kieżun, J.; Kraziński, B.E.; Kmieć, Z. Colorectal cancer patients exhibit increased levels of galanin in serum and colon tissues. Oncol. Lett. 2016, 12, 3323–3329.
- Kiezun, J.; Godlewski, J.; Krazinski, B.E.; Kozielec, Z.; Kmiec, Z. Galanin Receptors (GalR1, GalR2, and GalR3) Expression in Colorectal Cancer Tissue and Correlations to the Overall Survival and Poor Prognosis of CRC Patients. Int. J. Mol. Sci. 2022, 23, 3735.
- Talaat, I.; Yakout, N.; Soliman, A.; Venkatakhalam, T.; Vinod, A.; Eldohaji, L.; Nair, V.; Hareedy, A.; Kandil, A.; Abdel-Rahman, W. Evaluation of Galanin Expression in Colorectal Cancer: An Immunohistochemical and Transcriptomic Study. Front. Oncol. 2022, 12, 877147.
- Srivastava, A.; Rai, S.; Singh, M.P.; Srivastava, S. Computational Intelligence-Based Gene Expression Analysis in Colorectal Cancer: A Review. In Computational Intelligence in Oncology; Studies in Computational, Intelligence; Raza, K., Ed.; Springer: Singapore, 2022; Volume 1016, pp. 387–410.
- Singh, M.P.; Rai, S.; Singh, N.K.; Srivastava, S. Transcriptomic landscape of early age onset of colorectal cancer identifies novel genes and pathways in Indian CRC patients. Sci. Rep. 2021, 11, 11765.
- Kasprzak, A.; Adamek, A. The Neuropeptide System and Colorectal Cancer Liver Metastases: Mechanisms and Management. Int. J. Mol. Sci. 2020, 21, 3494.
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.-C.; Gugger, M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand 125I--bombesin(6–14). Clin. Cancer Res. 2002, 8, 1139–1146.
- Srivastava, A.; Rai, S.; Bisht, D.; Sachan, M.; Jit, B.P.; Srivastava, S. Targeting the altered tyrosine kinases in colorectal cancer: From inhibitors to drugs. In Protein Kinase Inhibitors: From Discovery to Therapeutics; Hassan, I., Noor, S., Eds.; Academic Press: London, UK, 2022; pp. 361–391.
- Sancho, V.; Di Florio, A.; Moody, T.W.; Jensen, R.T. Bombesin receptor-mediated imaging and cytotoxicity: Review and current status. Curr. Drug Deliv. 2011, 8, 79–134.
- Maoret, J.-J.; Pospaï, D.; Rouyer-Fessard, C.; Couvineau, A.; Laboisse, C.; Voisin, T.; Laburthe, M. Neurotensin receptor and its mRNA are expressed in many human colon cancer cell lines but not in normal colonic epithelium: Binding studies and RT-PCR experiments. Biochem. Biophys. Res. Commun. 1994, 203, 465–471.
- Wang, X.; Jackson, L.N.; Johnson, S.M.; Wang, Q.; Evers, B.M. Suppression of Neurotensin Receptor Type 1 Expression and Function by Histone Deacetylase Inhibitors in Human Colorectal Cancers. Mol. Cancer Ther. 2010, 9, 2389–2398.
- Iwase, K.; Evers, B.M.; Hellmich, M.R.; Kim, H.J.; Higashide, S.; Gully, D.; Townsend, C.M., Jr. Indirect inhibitory effect of a neurotensin receptor antagonist on human colon cancer (LoVo) growth. Surg. Oncol. 1996, 5, 245–251.
- Wang, X.; Wang, Q.; Ives, K.L.; Evers, B.M. Curcumin inhibits neurotensin-mediated interleukin-8 production and migration of HCT116 human colon cancer cells. Clin. Cancer Res. 2006, 12, 5346–5355.
- Briviba, K.; Abrahamse, S.L.; Pool-Zobel, B.L.; Rechkemmer, G. Neurotensin-and EGF-induced metabolic activation of colon carcinoma cells is diminished by dietary flavonoid cyanidin but not by its glycosides. Nutr. Cancer 2001, 41, 172–179.
- Hernandez, M.E.; Rembao, J.D.; Hernandez-Baltazar, D.; Castillo-Rodriguez, R.A.; Tellez-Lopez, V.M.; Flores-Martinez, Y.M.; Orozco-Barrios, C.E.; Rubio, H.A.; Sánchez-García, A.; Ayala-Davila, J.; et al. Safety of the intravenous administration of neurotensin-polyplex nanoparticles in BALB/c mice. Nanomedicine 2014, 10, 745–754.
- Levy, A.; Gal, R.; Granoth, R.; Dreznik, Z.; Fridkin, M.; Gozes, I. In vitro and in vivo treatment of colon cancer by VIP antagonists. Regul. Pept. 2002, 109, 127–133.
- Chen, X.-Y.; Ru, G.-Q.; Ma, Y.-Y.; Xie, J.; Chen, W.-Y.; Wang, H.-J.; Wang, S.-B.; Li, L.; Jin, K.-T.; He, X.-L. High expression of substance P and its receptor neurokinin-1 receptor in colorectal cancer is associated with tumor progression and prognosis. OncoTargets Ther. 2016, 9, 3595.
- Xiang, H.; Toyoshima, Y.; Shen, W.; Wang, X.; Okada, N.; Kii, S.; Sugiyama, K.; Nagato, T.; Kobayashi, H.; Ikeo, K. IFN-α/β-mediated NK2R expression is related to the malignancy of colon cancer cells. Cancer Sci. 2022, 113, 2513.
- Medeiros, P.J.; Al-Khazraji, B.K.; Novielli, N.M.; Postovit, L.M.; Chambers, A.F.; Jackson, D.N. Neuropeptide Y stimulates proliferation and migration in the 4T1 breast cancer cell line. Int. J. Cancer 2012, 131, 276–286.
- Chakroborty, D.; Goswami, S.; Fan, H.; Frankel, W.L.; Basu, S.; Sarkar, C. Neuropeptide Y, a paracrine factor secreted by cancer cells, is an independent regulator of angiogenesis in colon cancer. Br. J. Cancer 2022, 1–10.
- Couvineau, A.; Nicole, P.; Gratio, V.; Voisin, T. The Orexin receptors: Structural and anti-tumoral properties. Front. Endocrinol. 2022, 13, 931970.
- Messal, N.; Fernandez, N.; Dayot, S.; Gratio, V.; Nicole, P.; Prochasson, C.; Chantret, I.; LeGuilloux, G.; Jarry, A.; Couvelard, A. Ectopic expression of OX1R in ulcerative colitis mediates anti-inflammatory effect of orexin-A. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 3618–3628.
- Alain, C.; Pascal, N.; Valérie, G.; Thierry, V. Orexins/Hypocretins and Cancer: A Neuropeptide as Emerging Target. Molecules 2021, 26, 4849.
- Rouet-Benzineb, P.; Rouyer-Fessard, C.; Jarry, A.; Avondo, V.; Pouzet, C.; Yanagisawa, M.; Laboisse, C.; Laburthe, M.; Voisin, T. Orexins acting at native OX1 receptor in colon cancer and neuroblastoma cells or at recombinant OX1 receptor suppress cell growth by inducing apoptosis. J. Biol. Chem. 2004, 279, 45875–45886.
- Voisin, T.; El Firar, A.; Fasseu, M.; Rouyer-Fessard, C.; Descatoire, V.; Walker, F.; Paradis, V.; Bedossa, P.; Henin, D.; Lehy, T. Aberrant Expression of OX1 Receptors for Orexins in Colon Cancers and Liver Metastases: An Openable Gate to ApoptosisOrexin Receptor in Colon Cancers and Liver Metastases. Cancer Res. 2011, 71, 3341–3351.
