Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Physical
Photocatalysis is one of the most promising green technologies to utilize solar energy for clean energy achievement and environmental governance, such as artificial photosynthesis, water splitting, pollutants degradation, etc.
- photocatalysis
- assisted external physical fields
- coupling effects
1. Introduction
In order to achieve the carbon emission reduction target, photocatalysis has been widely considered to be a promising technology. After half a century of development, photocatalysis has tended to increasingly maturate, especially in many fields, such as pollutant degradation, water splitting, CO2 reduction, and desulfurization [1,2,3,4,5,6,7,8,9,10,11]. However, the main reason limiting photocatalytic technology from basic research to practical application is that the conversion efficiency of solar energy is still very low, which is far from meeting the requirements of commercial applications [12,13]. At present, there are two main ways to break the bottleneck of the efficiency of photocatalytic energy conversion: one is to enhance the absorption rate of the solar spectrum and the other is to efficiently promote the separation of photogenerated carriers.
Techniques for modification of photocatalytic materials, such as doping, compositing other materials, cocatalyst loading, morphology controlling, etc., have been confirmed to improve light absorption and photogenerated carrier separation efficiently [14,15,16,17,18]. However, the energy conversion efficiency is still very low although it has been improved to some extent by these modifications. Therefore, it is necessary to find a new way to achieve a breakthrough in photocatalytic performance. Studies have shown that photocatalytic activity can be greatly improved by adding other physical fields to the photocatalytic reaction process [19,20]. Generally, the assisted external field can provide additional energy to the photocatalytic system, which will power photogenerated charge separation and thereby improve the efficiency of photocatalysis. According to the energy form, as shown in Figure 1 the assisted physical field can be divided into the thermal field, mechanical field, and, electromagnetic field. In terms of energy generation types, it can also be divided into infrared thermal effect, microwave thermal effect, ultrasonic cavitation effect, friction electrostatic effect, external controllable electromagnetic field effect, and so on. One of the most common assisted physical fields is the thermal field. For example, in the photocatalytic reaction, most of the infrared light in the sunlight cannot be effectively used, which will dissipate into heat. Since infrared light and heat are inseparable, the infrared absorbing material can be composited into the photocatalytic material to collect the infrared band energy promoting the separation of photogenerated carriers. In addition, other non-contact external fields, such as the electric field, magnetic field, microwave field, and ultrasound field, can significantly improve the efficiency of the photocatalytic system, which have different mechanisms and respective advantages.
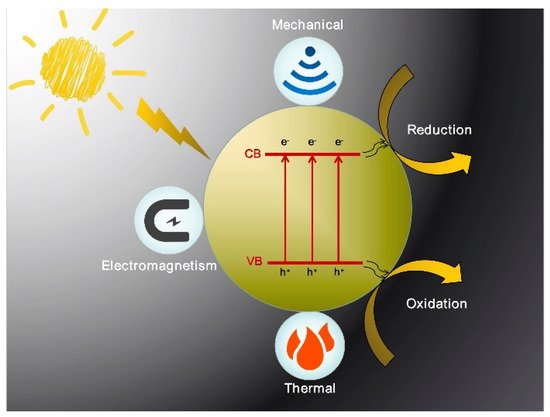
Figure 1. Schematic diagram of three types of external field-assisted photocatalysis.
2. Electromagnetism-Coupled Photocatalysis (ECP)
The electromagnetism-coupled photocatalysis is divided into two parts to review. They are electro-coupled photocatalysis and magnetism-coupled photocatalysis, respectively. In addition, Table 1 summarizes the application results of electromagnetism-coupled photocatalysis in recent years.
Table 1. Summary of electromagnetism-coupled photocatalysis in recent years.
| Material | Condition | Application | Activity | Blank Control | Ref. |
|---|---|---|---|---|---|
| ZnO | UV light magnet (600 mT) |
MB degradation |
DE = 60% (3 min) |
PC: DE = 24% |
[203] |
| CoFe2O4 | UV light (100 mW/cm2) magnet (200 mT) |
MB degradation |
DE = 80% (60 min) |
PC: DE = 28% |
[204] |
| n-TiO2/PS | 400 W UV light power supply (3 V) |
MB degradation |
DE = 38% (30 min) |
PC: DE = 20% |
[205] |
| rGO/TiO2 | 40 W UV lamp Nd2Fe14B magnet |
MO degradation |
DE = 91% (120 min) |
TiO2: DE = 68% |
[206] |
| TiO2 | 4 W mercury lamp magnet (280 mT) |
MO degradation |
DE ≈ 94% (75 min) |
PC: DE ≈ 70% |
[207] |
| α-Fe2O3/TiO2 | Diode Green Laser magnet (400 mT) |
RhB degradation |
DE ≈ 70% (60 min) |
PC: DE ≈ 45% |
[208] |
| Ti0.936O2 | 300 W Xe lamp electromagnet (80 mT) |
RhB degradation |
k ≈ 0.075 min−1 | PC: k ≈ 0.05 min−1 |
[209] |
| BiFeO3 | 500 W Xe lamp (λ > 420 nm) electrical poling |
RhB degradation |
k = 0.035 min−1 | PC: k = 0.016 min−1 |
[210] |
| α-Fe2O3/rGO | 300 W Xe lamp magnet |
CR degradation |
DE = 87% (30 min) |
PC: DE = 60% |
[211] |
| CoFe2O4/MoS2 | 300 W Xe lamp electromagnet (150 mT) |
CR degradation |
DE = 96.6% (60 min) |
PC (50 mT): DE = 82% |
[212] |
| BiOBr/BNQDs | 300 W Xe lamp (λ > 420 nm) magnet |
TC degradation |
DE = 81% (60 min) |
BiOBr: DE = 60% |
[213] |
| hierarchical TiO2 microspheres |
500 W Xe lamp power supply (2 V) |
TBT degradation |
k = 0.0488 min−1 | PC: k = 0.0052 min−1 |
[214] |
| Mn3O4/γ-MnOOH | 300 W Xe lamp magnet (60 mT) |
NOR degradation |
DE = 98.8% (160 min) |
PC: DE = 90.3% |
[215] |
| α-Fe2O3/Zn1-xFexO | Xe lamp (λ > 420 nm) ten magnets (20 mT) |
RIB degradation |
k = 0.0125 min−1 | PC: k = 0.0072 min−1 |
[216] |
| Au/Fe3O4/N-TiO2 | 70 W tungsten light magnet (180 mT) |
H2 production | 21230 μmol g−1 h−1 | PC: 7600 μmol g−1 h−1 |
[217] |
| CdS/MoS2/Mo | 300 W Xe lamp (100 mW/cm2) rotating magnet |
H2 production | 1.97 mmol g−1 h−1 | PC: 1.04 mmol g−1 h−1 |
[218] |
| Au-CdS | 300 W Xe lamp rotating magnet |
H2 production | 105 μmol g−1 h−1 | PC: 223 μmol g−1 h−1 |
[219] |
| Pt/TiO2 | 3 W LED lamp DC power (1 V) |
H2 production | 3242.6 μmol g−1 h−1 | PC: 1102.8 μmol g−1 h−1 |
[220] |
| K0.5Na0.5NbO3 | 300 W Xe lamp corona poling (690 kV/cm) |
H2 production | 470 μmol g−1 h−1 | PC: 63 μmol g−1 h−1 |
[221] |
| Rutile TiO2 nanograss | sunlight power supply (2 V) |
Cr ion removal | 143.8 mg/g | - | [222] |
| Bi2MoO6 | 300 W Xe lamp corona poling (20 kV/cm) |
CO2 reduction | 14.38 μmol g−1 h−1 (CO) |
PC: 4.08 μmol g−1 h−1 |
[223] |
| BaTiO3 | 300 W Xe lamp magnet |
nitrogen fixation | 1.93 mgL−1 h−1 | PC: 1.35 mgL−1 h−1 |
[224] |
2.1. Electro-Coupled Photocatalysis
Adding an external electric field to the photocatalytic system can effectively promote the separation and migration of photogenerated carriers. At present, there are two main ways for researchers to add an electric field: one is to directly connect to an external power source during the photocatalytic process, and the other is to polarize the catalyst in the electric field and then perform photocatalytic experiments.
Directly adding an external electric field during the photocatalytic process is a common utilization method in electro-coupled photocatalysis. Li et al. synthesized rutile TiO2 nanograss on titanium mesh [222]. By connecting the titanium mesh to a power source, the photocatalytic removal capacity of rutile TiO2 nanograss for Cr(VI) ions in wastewater can reach 143.8 mg/g under an external electric field and sunlight. Studies have shown that electrons provided by an external electric field can effectively inhibit the recombination of photogenerated carriers, thereby enhancing the catalytic activity. Pan et al. synthesized layered TiO2 microspheres for the removal of tributyltin (TBT) in electro-coupled photocatalysis, and the reaction setup is shown in Figure 2 [214]. The bias potential provided by the applied electric field greatly facilitates the separation of photogenerated carriers. The results show that the removal reaction rate constant (k = 0.0488 min−1) under electro-coupled photocatalysis is nearly times higher than that under photocatalysis (k = 0.0052 min−1). Zhang et al. developed a new electro-assisted photocatalytic technology for liquid and gas phase reactions (such as water splitting and CO2 reduction) [220]. They applied a lower external voltage on the self-doped TiO2 nanotube films. With electrical assistance, the recombination of photogenerated carriers is greatly suppressed, and photoexcited electrons are ejected from the catalyst, enabling spatial separation of carriers. It is worth mentioning that the photocatalytic redox reaction is integrated on a thin film in this system, rather than taking place on traditional separate electrodes.
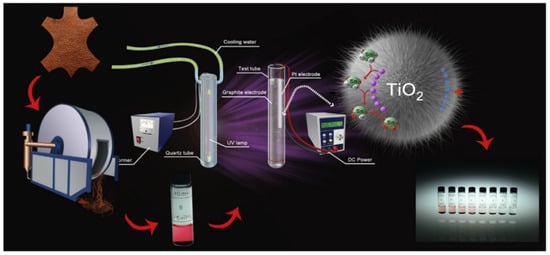
Figure 2. Diagrammatic sketch of the electro-field-assisted-photocatalytic system (Reprinted/adapted with permission from Ref. [214]. Copyright 2017, copyright Elsevier).
In addition to directly adding an external electric field, the researchers also developed another way of electrically coupled photocatalysis. They put the ferroelectric material into an external electric field for polarization before conducting the photocatalytic experiments. The strong ferroelectric field formed by the polarized material can effectively promote charge separation and improve photocatalytic efficiency. Nam et al. prepared polarized K0.5Na0.5NbO3 powder by a corona polarization method for H2 production [221]. The enlarged positively charged surface of the catalyst promotes the photocatalytic activity. Under optimal conditions, its photocatalytic activity was increased by 7.4 times. Yun et al. prepared well-polarized BiFeO3 nanoparticles by a simple electrical polarization method [210]. The photocatalytic degradation rate of RhB by the polarized BiFeO3 nanoparticles was increased by two times compared with the unpolarized. Its excellent photocatalytic performance can be attributed to the improved carrier separation by ferroelectric polarization. Tian et al. prepared ultrathin Bi2MoO6 nanosheets with strong ferroelectricity by a corona polarization method for photocatalytic CO2 reduction [223]. Its CO generation rate is 14.38 μmol g−1 h−1, which is more than 10 times higher than that of bulk Bi2MoO6. As shown in Figure 3, this combined strategy significantly promotes the separation of photogenerated electrons and holes, enriches the reaction sites for CO2 adsorption, and jointly improves the photocatalytic CO2 reduction performance.
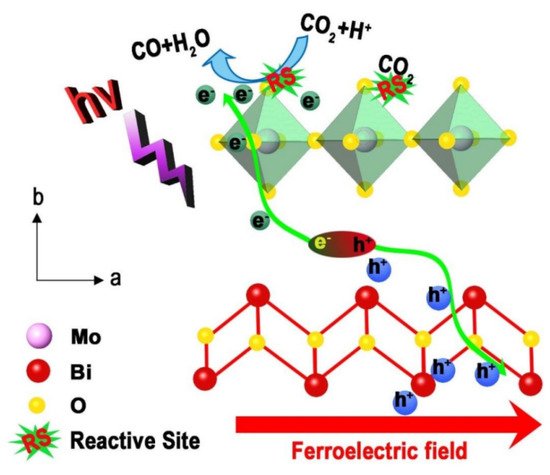
Figure 3. Schematic diagram of ferroelectric field-assisted photocatalysis of Bi2MoO6 (Reprinted/adapted with permission from Ref. [223]. Copyright 2020, copyright Royal Society of Chemistry).
2.2. Magnetism-Coupled Photocatalysis
In recent years, more and more attention has been paid to the promotion of magnetic fields on photocatalytic performance. In a magnetism-coupled photocatalytic system, the magnetic-field-induced Lorentz force diverts the charge from its original recombination path, leading to more carriers participating in the reaction, thereby enhancing the photocatalytic activity [206,213,216,225,226]. In addition, for materials with electron spin properties, the magnetic field can also enhance the electron-spin parallel alignment, which also has a positive effect on photocatalytic performance [209,217,227].
Gao et al. reported a Lorentz force-enhanced photogenerated carrier separation strategy using highly crystalline TiO2 nanosheets with few defects as photocatalysts [225]. Studies have shown that the Lorentz force suppresses photogenerated carrier recombination, making more carriers available for transport in photocatalysts. The results showed that the photocatalytic degradation rate of MO was increased by 26% only by placing permanent magnets under the photocatalytic reactor. Nan et al. synthesized a 3D/2D Mn2O3/g-C3N4 photocatalyst for simultaneous removal of nitrate and ammonia from polluted water and developed a magnetic field-enhanced photocatalytic system [226]. Under the action of an external magnetic field, the removal efficiencies of nitrate and ammonia were 94.5% and 97.4%, respectively. The external magnetic field promotes the separation of carriers by generating the Lorentz force. Tsang et al. reported a photocatalytic water splitting system based on Fe3O4/N-TiO2 magnetic photocatalyst assisted by the local magnetic field effect [217]. At a magnetic field strength of 180 mT and 270 °C, the quantum efficiency at 437 nm reaches 88.7%. As shown in Figure 4, the synergistic effect of the Lorentz force and spin polarization greatly facilitates the charge separation process, thereby enhancing the photocatalytic efficiency. Sang et al. prepared a CdS/MoS2/Mo hybrid structured photocatalyst and studied the effect of magnetic field on photocatalytic activity [218]. The relative motion between the metal molybdenum sheet and the rotating magnetic field forms a kinematic electromotive force. This electromotive force significantly inhibits the photo-induced carrier recombination of CdS. Under the synergistic effect of MoS2 co-catalyst, its photocatalytic H2 production performance is improved by approximately 89% compared with single photocatalysis. Chen et al. synthesized a Mn3O4/γ-MnOOH photocatalyst for norfloxacin (NOR) degradation [215]. Under the action of magnetic field-assisted visible light, the degradation rate of NOR was 98.8% within 60 min. In neutral media, positively charged NOR and negatively charged catalysts align in the presence of a magnetic field, thereby enhancing the reactivity. Furthermore, the opposing Lorentz force contributed to the mutual attraction between NOR and catalyst, which accelerated NOR degradation.
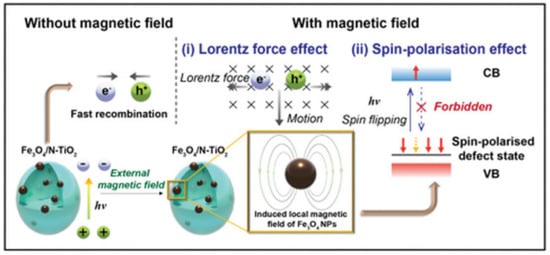
Figure 4. Schematic diagram of magnetic field-promoted photocatalytic water splitting system (Reprinted/adapted with permission from Ref. [217]. Copyright 2022, copyright Royal Society of Chemistry).
This entry is adapted from the peer-reviewed paper 10.3390/catal12091042
This entry is offline, you can click here to edit this entry!
