Owing to the low efficient technology on the production, storage, and operation of hydrogen, pure hydrogen fuel supply is still not an economical and effective way to support the transportation system. Using hydrogen-rich fuel to provide hydrogen is a good means, which is relatively safe, efficient, and economical. At this time, ammonia received attention. Ammonia is considered as an excellent hydrogen-rich fuel with potential application prospects in low-carbon energy storage, transportation, and power generation. Because it can promote agricultural production, people realized the important role of ammonia long ago. A century ago, the emergence of synthetic ammonia technology greatly expanded the application of ammonia. With the development and optimized utilization of this carbon-free compound, ammonia energy established a well-developed production and distribution infrastructure system. Since the inconvenient storage and transfer system of hydrogen hinders the feasibility of its practical development, ammonia has attracted the attention of researchers because of its advantages, such as easy liquefaction, sound transfer system, and excellent development potential.
2. Production, Storage and Transportation of Hydrogen and Ammonia Energy
2.1. Production, Storage and Transportation of Hydrogen Energy
Although hydrogen is widely distributed in nature, it usually occurs in the form of a compound called hydride that has negative or anionic characteristics and is not readily available. Many research studies have focused on realizing different methods for hydrogen production. The primary purpose of these studies is to produce hydrogen at a low cost with high efficiency. Based on the direct energy and technology of hydrogen production, hydrogen can be divided into different colors, e.g., grey, blue, turquoise, green, purple, and yellow [
31]. Currently, the three most discussed hydrogen colors are green, blue, and grey.
2.1.1. Production of Hydrogen
Hydrogen reformed from fossil fuel is the most widely used hydrogen production technology. Steam methane reforming is also a widely used method at present, with conversion efficiency ranging from 74% to 85% at a low production cost. Among all current hydrogen production technologies, electrolysis water hydrogen production technology is relatively mature in industrial applications [
32]. The electricity in this process can be powered by renewable energy sources and obtained through low-carbon or carbon-free methods. Using renewable hydrogen production is a hot research topic in new energy sources, including wind hydrogen [
33], solar water splitting to hydrogen, nuclear hydrogen production, and hydrogen production from biomass. Storing energy in the form of hydrogen is a promising alternative to green energy; if hydrogen is to realize its potential to be an energy vector in a decarbonized economy, it needs to be produced on a sustainable scale [
34]. Biomass power generation realizes the utilization of organic waste and hydrogen production through biochemical action. Due to its low production efficiency, it is still limited to laboratory-scale research.
Grey hydrogen generally refers to hydrogen made from fossil fuels, such as oil, natural gas, and coal. The hydrogen produced by adding carbon capture and storage (CCS) to grey hydrogen is called blue hydrogen, which effectively reduces carbon emissions in the production process. However, the utilization of CCS will lead to a dramatic increase in operating costs. At the same time, methane leakage (from natural gas) that occurs during blue hydrogen production cannot be ignored [
35,
36]. Green hydrogen is hydrogen produced by the electrolysis of renewable energy sources, which not only emits no carbon dioxide, but employs an efficient way to store energy and can help solve the intermittent problems of plaguing wind and solar power [
32]. According to data from The International Energy Agency, by 2030, wind energy and hydrogen energy generated by electrolysis will be cheaper than natural gas [
37]. Green hydrogen currently costs between $2.70–$8.80/kg to produce globally with all studies projecting a sharp drop to $2~$6/kg by 2030 [
38]. Purple hydrogen is obtained by electrolysis through an atomic current. Attaching a hydrogen production facility can provide a further energy storage possibility once seasonal storage might be required [
31].
2.1.2. Storage of Hydrogen
For vehicle applications, weight density and volume density need to be considered together to ensure that there is enough hydrogen to travel a reasonable distance without refueling the vehicle. Therefore, it is necessary to provide both high weight energy density and high-volume energy density storage methods [
39], and the energy storage technology should obtain the following characteristics: high hydrogen storage capacity, rapid reaction kinetics, high reversibility, reasonable cost and safety [
40]. At present, the onboard hydrogen storage technology of hydrogen fuel cell vehicles mainly includes high-pressure gaseous hydrogen storage, low-temperature liquid hydrogen storage, high-pressure and low-temperature liquid hydrogen storage, metal hydride hydrogen storage, and organic liquid hydrogen storage, etc.
High-pressure gaseous hydrogen storage is a widely used and relatively mature technology. It is the mainstream hydrogen energy storage and transportation mode. Compressed hydrogen can be stored in high-pressure gas cylinders and divided into type I, type II, type III, and type IV. For type III, because of the low thermal conductivity which may cause problems with the low heat release rate during hydrogen compression, they are suitable for operating at 450 bar pressures. For type IV, they are made entirely of composite materials, the liner of which is mostly polymeric as that of high-density polyethylene (HDPE) and are also used to store hydrogen at 700 bar pressure. However, pressurized hydrogen storage may lead to hydrogen embrittlement [
44].
Medium- and low-temperature liquid hydrogen storage must liquefy the hydrogen first. The volume energy density of liquid hydrogen is high, and the density of liquid hydrogen reaches approximately 71 g/L at −253 °C [
41]. Hydrogen has an extremely low volumetric energy content, which is 0.01 MJ/L H
2 at ambient conditions and 8.5 MJ/L H
2 for the liquefied H
2 (LH
2) [
42]. The volume energy density of liquid hydrogen is high, but the liquefaction process consumes high-level energy and requires a liquefaction tank with good thermal insulation performance. The material requirements are strict. Because of the extremely low temperature conditions and evaporation phenomenon, liquefied hydrogen is difficult to use in vehicles, especially in small- and medium-sized vehicles [
45].
Organic liquid hydrogen storage uses organic liquid compounds such as methane and other aromatic organic compounds to fix hydrogen through a hydrogenation reaction, so as to form liquid compounds with hydrogen bound in molecules to realize the function of hydrogen storage. The organic hydrides are dehydrogenated in a reactor to provide fuel for the vehicle. The traditional organic hydrogen storage technology has a reaction pressure of 1–10 mpa and a reaction temperature of about 350 °C, which requires precious metal catalysts. The organic hydrides have a fairly high reversible hydrogen capacity, ranging from 1.7 to 7.3 wt%, and are liquid at room temperature. The greatest advantage of using organic hydrides is the efficient use of current gasoline bulk storage and the transportation infrastructure [
46].
Hydrogen storage by metal hydride [
43] absorbs hydrogen in form of all hydrides through the combination reaction between hydrogen and metal, which can provide higher hydrogen storage capacity than compression and liquefication [
47], and store hydrogen at moderate temperature and pressure with large volume density and good reversible cycle. The hydrogen provided in this method is a safe, reliable and of high purity, and is more suitable for automotive fuel cells. It is a kind of solid hydrogen storage method developed rapidly in recent years and has received public attention in the application of hydrogen. However, the drawback of this technology is that most metal hydrides cannot provide storage for large amounts of hydrogen. However, this cannot affect that solid-state hydrogen storage is at the stage of rapid development and will be a research hotspot in the future for a long time.
2.1.3. Transportation of Hydrogen
Hydrogen transportation is a critical link in the hydrogen energy industry chain and is vital to optimizing the geographical allocation of hydrogen sources. The hydrogen transportation mode is mainly determined by its storage form. There are three main transmission methods [
48]: gaseous hydrogen delivery, liquid hydrogen delivery and hydrogen carriers (material based). The energy density and bulk density of liquid hydrogen storage is higher than that of gaseous hydrogen storage. Although, it is technically complex and costly because it needs to be stored in low-temperature insulated containers.
Gaseous hydrogen can be transported by a long-distance trailer or long-distance pipeline after being pressurized. Hydrogen-mixed natural gas hydrogen transmission technology is a new scenario for hydrogen transmission proposed by scholars in recent years. Using hydrogen and natural gas mixed-gas for transportation refers to adding a specific concentration of hydrogen into the natural gas pipeline system, but the hydrogen and natural gas transported by steel pipes or iron pipes can leak in threads or mechanical joints; the width of the pipe diameter and the concentration of hydrogen have an impact on the leakage rate of the mixed gas [
44].
2.2. Production, Storage and Transportation of Ammonia Energy
Ammonia has been widely used as a precursor of nitrogen-containing compounds such as agricultural fertilizers and pharmaceuticals. Due to the high hydrogen density (17.65 wt%) and easy liquefaction characteristics (8.88 bar, 21 °C liquefaction) of ammonia, the use of NH
3 as a hydrogen carrier has been investigated since the 1960s [
49]. Based on the famous Haber–Bosch process and the broad global large-scale production distribution, the involvement of ammonia provides advantages for the large-scale use of existing facilities to store/transport hydrogen.
2.2.1. Production of Ammonia
At the beginning of the 20th century, the Haber–Bosch process (hereinafter referred to as the H–B process) successfully commercialized the production of ammonia. After a century of development, the H–B process has evolved into a more environmentally friendly generation. At the same time, there are also various emerging methods to produce ammonia energy.
There are three ammonia production methods with high technical maturity: The H–B process, ammonia production from renewable energy and the electrochemical synthesis of ammonia.
- (1)
-
H–B process is the synthesis of ammonia by hydrogen and nitrogen under the action of a catalyst at high temperature and high pressure. The H–B process is employed as the mainstream ammonia production method today. Many carbon emissions will be generated in the synthesis process due to the generation mode of raw material hydrogen (gray hydrogen), so it is also called gray ammonia. Ammonia production currently accounts for 1.0% of global greenhouse gas emissions [
50]. Similar to the classification of hydrogen energy, when blue hydrogen introduced into carbon capture technology (CCUS) is used to produce ammonia, the product is called blue ammonia;
- (2)
-
In essence, hydrogen production from renewable energy still adopts the H–B process. Because the hydrogen at source is derived from renewable production (green hydrogen), the ammonia produced in this way is also called green ammonia. It is worth mentioning that green ammonia provides more clean options on the supply side as a fuel and has great potential as a fixed carrier and hydrogen transport carrier of renewable energy (wind energy, solar energy, etc.);
- (3)
-
Electrochemical ammonia synthesis uses electrochemical means to synthesize ammonia from nitrogen molecules and hydrogen in H2O through a redox medium. This method has lower requirements for the purity of raw materials than other methods, which has a high intermittence tolerance to the reaction. Therefore, the electrochemical synthesis method is restricted when taking the total carbon and nitrogen oxide emissions cycle into consideration.
In addition to the three methods mentioned above, some are still in the early stage of experiments, such as the photoreduction N
2 method [
52] and the biotechnology approach [
53]. Due to technical constraints, the output of these methods is hard to be enlarged and the production process is difficult to be stable. At present, they are in a period of technical pre-maturity and provide new research directions to reduce nitrogen oxide pollution and synthetic ammonia.
2.2.2. Storage and Transportation of Ammonia
In transportation, ammonia is often used as the storage and transportation carrier of hydrogen. Ammonia is easy to liquefy and becomes liquid when cooled to −33.34 °C (239.75 K) under normal pressure or pressurized to 700–800 kpa under average temperature. It is easier to store and transport. In particular, compared with other hydrogen storage methods, the advantage of ammonia lies in its perfect global distribution network, treatment methods, and policies and laws on storage and transportation.
There are three main modes of transportation for liquid ammonia: pipeline network transport, tanker transport, and cruise/ship transport. Different transport modes can provide different ranges and capacities and afford more options for the ammonia energy market. Pipeline transport can reduce the cost by transforming other liquid transmission networks; tanker transport tends to meet the demand of short-distance supply, while cruise/ship transport can realize long-distance and large-scale transportation across the oceans.
Due to the risk of leakage during the transportation of liquid ammonia, solid-state storage has become a research hotspot in recent years, the principle of which is to fix ammonia in solid form by combining ammonia with metal amine complexes. Metal amines have low toxicity and can be desorbed at a lower temperature. The desorption of Ca (NH3) 8Cl2 can be carried out at a lower temperature of about 60 °C, and the ammonia vapor pressure at room temperature is as high as 0.7 bar.
Solid-state ammonia is notable for overcoming the issues associated with the liquid storage of ammonia, and many scientific studies indicate that several metal salts have shown better ammonia storage capacity. Several materials, such as metal halides, borohydrides, and proton-based materials, are used industrially for ammonia removal caused by leaks out of refrigeration pipelines. This approach has led to the utilization these materials as ammonia carriers without a cooling system [
54]. Solid-state storage should be a key development topic in the future.
3. Road Transportation Application form of Hydrogen Energy
There are two potential pathways for the application of hydrogen in automobiles. One is to use hydrogen as a fuel for engines, and the other is to generate electricity through fuel cells to drive cars and other electrical appliances [
55].
3.1. Hydrogen Fuel Engine
The only product of the complete combustion of hydrogen fuel is water without producing smoke. The combustion reaction of hydrogen generated inside the engine can be described as Formula (1):
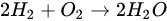
Hydrogen engines can adopt a higher compression ratio to improve their thermal efficiency because of the high spontaneous combustion point of hydrogen. The combustion state of hydrogen in the cylinder is closer to the ideal state, and its flame propagation speed is fast. Compared with a gasoline engine, it has the advantage of high thermal efficiency [
56]. Hydrogen engine vehicles, which were developed by BMW iX5 Hydrogen of Germany, Toyota of Japan, and Changan of China, and have further proved that hydrogen engines have good dynamic performance and low carbon emissions potential [
57,
58]. Therefore, using hydrogen energy as the engine energy supply to replace fossil fuel energy is also a research hotspot in the current automobile industry.
The intake structure of a hydrogen engine mainly includes direct injection (DI) and port fuel injection (PFI). PFI hydrogen engine is easy to refit from existing machines and has a good economic performance. PFI hydrogen engine plays a leading role in hydrogen engines developed by various countries. However, it is prone to premature combustion, tempering and other abnormal combustion phenomena in combustion process, which leads to a relatively lower output power. DI hydrogen engine can overcome the above problems and effectively avoid abnormal combustion and controllable emissions. Furthermore, it can significantly improve the output power performance, which is the focus of current and future research or development directions.
A sharp increase in cylinder pressure resulting by the rapid combustion of hydrogen and the problems existing in the engine, including deflagration, early combustion, and tempering, are technical bottlenecks to promote the performance of hydrogen engines.
3.2. Hydrogen Fuel Cell
Hydrogen fuel cells are different from lithium–ion batteries [
59] and supercapacitors [
60] in storing electric energy. Since the basic principle is to provide power through electricity conversion, hydrogen fuel cell vehicle has apparent advantages such as short fuel filling time and long driving range, which can effectively compensate for the inherent disadvantages of pure battery electric cars in medium and long-distance transportation. It is one of the essential directions for developing new energy vehicles in the future.
Typical fuel cells can be divided into proton exchange membrane fuel cell (PEMFC), solid oxide fuel cell (SOFC) phosphoric acid fuel cell (PAFC) [
61] and alkaline fuel cell (AFC) according to their electrolytes. PEMFC can quickly reach working status, it has stable working pressure due to its relatively simple working mechanism and can use the electrolyte without involving complex chemical changes. It also is regarded as a dominant choice in the automotive fuel cells compared to the others. Still, the high cost of proton exchange membrane fuel cell systems and the poor service life are key issues for preventing fuel cell car large-scale industrialization [
62]. SOFC is an all-solid-state chemical power generation device, which can directly convert the chemical energy stored in fuel and oxidant into electric energy, and environmentally friendly at medium and high temperatures. The high working temperature (700~900 °C) of SOFC leads to severe defects in its overall performance and durability, which limits its applications [
63,
64]; AFC takes an alkaline aqueous solution as an electrolyte, which is inexpensive to produce hydrogen but poses health risks due to the adoption of asbestos [
65].
The hydrogen fuel cell does not require the burning of fuel to acquire energy. The hydrogen enters the fuel cell’s anode (hydrogen electrode) and reacts with the catalyst covered on the anode to release electrons and forms positively charged hydrogen ions. Electrons flow into the circuit to create current and generate electric energy. Compared with pure battery electric vehicles, which extend their range by adding battery packs, fuel cell vehicles extend their content by adding hydrogen storage. It is cheaper to add hydrogen storage to extend the range of fuel cell vehicles than to add battery packs to extend the range of pure battery electric vehicles, thereby making them more cost-competitive at distances more than 300 km. In long-distance driving, compared with pure battery electric vehicles, the charging time of fuel cell vehicles can achieve as short as 5 min. However, since the cost of core technologies of fuel cells such as proton exchange membranes is still high and the technical level cannot reach the level of commercialization, it is very difficult to promote hydrogen fuel cells. At present, most countries are still at the stage of financial support from the state and the technology cannot be commercialized. This step may need to be solved by a fundamental technological development. Even so, hydrogen technology still has the most potential to promote the reform of urban air pollution and fuel cell technology simultaneously.
4. Road Transportation Application Mode of Hydrogen and Ammonia
4.1. Application Mode of Hydrogen Energy Highway
Compared with traditional locomotives, hydrogen fuel cell vehicles have the advantages of environment protection, shorter hydrogenation time and longer mileage.
4.1.1. Application Mode of Hydrogen Fuel Engine
In 2005, BMW launched the “H
2R” hydrogen-powered vehicle at Shanghai International Auto Show. BMW launched the BMW hydrogen7 sedan powered by hydrogen in 2007 [
70]. In 2009, BMW launched the “h2bvplus” hydrogen engine vehicle with the maximum thermal efficiency exceeding 40%. Ford [
71] of the United States and Mazda of Japan also launched their own hydrogen engine vehicles, demonstrating the feasibility and advantages of hydrogen as engine fuel.
However, the high temperature of hydrogen during combustion and the fast flame propagation speed leads to a large amount of NO
x emission during the operation of a hydrogen engine. Hence, reducing NO
x emissions is an important issue to promote the development of hydrogen engines. Through the study of single-cylinder engines, C.l. Park [
72] verified that as the direct injection fuel injection timing is retarded, the intake airflow rate increases owing to the combustion of the relatively lean mixture. The emission of NO
x decrease as the excess air ratio increases. K.A. Subramanian et al. [
73] reduced NO
x emission of the engine from the source by using exhaust gas recirculation (EGR) and water injection techniques to control combustion. The experimental results show that both methods effectively control tempering and NO
x emission. But water injection technology can prevent tempering more effectively than the EGR technique.
4.1.2. Application Mode of Hydrogen Fuel Cell
In February 2013, the world’s first mass-produced hydrogen fuel cell vehicle was manufactured by Hyundai Motor of South Korea, which is the world’s first ready-made hydrogen fuel cell vehicle group. In 2014, Toyota’s first-generation Mirai went on sale. According to the announcement of Toyota China in 2019, the cumulative sales of Mirai in the world reached 10,000. The development route of hydrogen fuel vehicles in China is different from that of any other automobile manufacturer such as Toyota and Hyundai. Considering heavy trucks as the primary source of greenhouse gas emissions in the transportation fleet, China is focusing on the development of hydrogen-powered trucks and buses. In terms of buses, China has a large deployment scale. By the end of 2018, more than 400 buses had been registered for demonstration projects [
74]. The French hydrogen strategy plans to produce 20,000–50,000 light vehicles, 800–2000 heavy vehicles and 400–1000 hydrogen gas stations by 2028 [
75].
Quantron proposed to produce hydrogen fuel cell logistics vehicles in 2020 [
80], in which the output power of 4.2-ton hydrogen fuel cell vehicles is 100 kW, and the output power of 7.2-ton fuel cell vehicles is 147 kW, with a range of 300–500 km. In 2021, the Yanshan Petrochemical Hydrogen Energy Heavy Haul Truck [
81] was launched in Beijing and put into short-distance transportation, setting a precedent for the demonstration and application of hydrogen energy heavy trucks in Beijing. In 2018, Zhengzhou public transport used two Yutong buses [
82] for the operation demonstration; Yutong Bus also delivered a hydrogen bus for the Yanqing competition area of the Olympic Winter Games Beijing 2022. To cope with the extreme weather conditions during the Olympic Winter Games, the hydrogen bus equipped with the FCS80 fuel cell system that can be started at a low temperature of minus 30 °C.
4.2. Ammonia Energy Highway Application Mode
4.2.1. Application Mode of Ammonia Fuel Engine
As early as the 1960s, the idea of using ammonia as fuel has emerged and has been preliminarily studied. Up to now, the research direction of ammonia as fuel is mainly focused on the following areas: using ammonia as a combustion promoter to assist the combustion of primary fuels, optimizing the fuel ratio to improve combustion efficiency, and reducing tail gas and tail gas emission treatment strategies.
- (1)
-
Using ammonia as auxiliary fuel can improve the working efficiency of traditional engines and effectively reduce NOx emission. Ammonia can also be used as the primary fuel. Through the hydrogen produced by the early decomposition of ammonia, the combustion speed can be accelerated, and the availability of an ammonia fuel engine can be improved.
Lee et al. [
83] proposed a combustion strategy that employed ammonia itself as a combustion promoter, which used pure ammonia as engine fuel and converted stored energy into usable forms. Under the strategy, the conditions of high temperature and high pressure were sufficient to cause the combustion of ammonia spray obtained through the spontaneous combustion of ammonia mixture by pilot injection. The NO
x generation of the dual-fuel engine was analyzed in stages. Xu et al. [
84] studied various feasible methods and directions of ammonia decomposition to produce hydrogen before being used as fuel. Lamas et al. [
85] analyzed the technology of injecting ammonia directly into the combustion chamber and carried out numerical research on the compression ignition engine with mixed hydrogen diesel fuel. The results show that the formation of NO
x can be effectively reduced by adjusting the flow shape and injection time of ammonia injection.
- (2)
-
Aiming at the incomplete combustion of ammonia that cannot be ignored, how to deal with the tail gas has also become a research hotspot. The unified way is to install post-treatment devices for engines [
86].
A thermochemical recovery (TCR) reactor was developed in reference [
87] to improve the general exhaust gas treatment device, and the experimental study was carried out. The fuel adopted hydrogen ammonia mixed energy that could oxidize the unburned ammonia in the tail gas, and then discharged it. The results showed that ammonia provides up to 55% of the total fuel energy based on its low calorific value. When the exhaust temperature was high, the ammonia decomposition conversion rate in the TCR reactor was high and the overall braking thermal efficiency of the engine was improved. However, the ammonia decomposition conversion rate of other operation modes was reduced because of the poor combustion efficiency. Since the ratio of NH
3/NO
x in engine exhaust is suitable for passive selective catalytic reduction, when appropriate catalysts are used in the TCR reactor, ammonia and NO
x in the engine can be easily converted to N
2. The problem of pollutant emission is not a trivial matter and needs in-depth research to put forward optimization solutions according to the best operation mode to solve the problems of NO
x and NH
3 or even H
2. As Angeles et al. [
88] pointed out, any vehicle ammonia fuel cycle optimization might be affected by the “limited data of ammonia fuel vehicle performance”.
4.2.2. Application Mode of Ammonia Fuel Cell
In the early 21st century, the research on ammonia fuel cells has begun to show signs [
89]. The applications mainly focus on solid oxide cells (SOFC) and direct ammonia fuel cells (DFAC).
- (1)
-
Solid Oxide Cells (SOFC)
Compared with other fuel cells, SOFC has many advantages, of which NH
3 reacts electrochemically and thus avoiding the generation of typical combustion pollutants such as NO
x. Mariagiovanna et al. proposed a gas station model for supplying hydrogen or electricity to vehicles by combining ammonia and SOFC, and discussed the economy of this form. The results show that the values of the LCOH (levelized cost of hydrogen), for the proposed configurations and economic scenarios, are in the range of 6–10 €/kg and the values of the LCOE (levelized cost of electricity) range from 0.447 €/kWh to 0.242 €/kWh [
90]. Although the performance of SOFC can be compared with that of the hydrogen fuel cell, its stability is reduced due to the anode degradation which occurs from the deformation of microstructure caused by the formation of nickel nitride in the anode. Adding ammonia to SOFC can effectively alleviate the nitride reaction between ammonia and nickel. Only a gaseous mixture of H
2 and N
2 can enter the SOFC and eliminate the reaction between ammonia and anode;
- (2)
-
Direct ammonia fuel cells (DAFC)
In recent years, low-temperature direct ammonia fuel cells (DAFC) have also attracted more and more attention, especially in automotive applications. Compared with high-temperature DAFC, low-temperature natural ammonia fuel cell has the advantage of a fast start-up. After years of development, the efficiency and power output of DAFC has been significantly improved. A notable challenge of DAFC is the ammonia-crossing chemical reaction, which is inevitable for ion-exchange membrane fuel cells and results degradation of cell efficiency and battery power [
91]. Chen et al. [
92] compared the performance of different DAFC with PtIr/C, PtRu/C, and Pt/C as anodes under mild conditions. The results showed that PtIr/C anodes could be used as commercial anode materials for DAFC.
Ezzat et al. [
93] compared and analyzed two types of onboard application integration systems using ammonia as energy supply. The first system included liquefied ammonia tank, dissociation and separation device (DSC) for decomposing ammonia and engine (ice) to power the vehicle. The second system was a hybrid system composed of liquefied ammonia tank, DSC unit, small ice, and fuel cell system. It was mainly powered by a fuel cell and assisted by the engine. Energy efficiency shows that the integration of fuel cells and engine improves the efficiency of the vehicle power system compared with vehicles using only the engine as its energy source. However, improving efficiency may lead to a dramatic cost increase in the power system.