Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Contamination of agricultural products and foods by aflatoxin B1 (AFB1) is becoming a serious global problem, and the presence of AFB1 in edible oil is frequent and has become inevitable, especially in underdeveloped countries and regions. As AFB1 results from a possible degradation of aflatoxins and the interaction of the resulting toxic compound with food components, it could cause chronic disease or severe cancers, increasing morbidity and mortality.
- aflatoxin B1
- edible oil
- chromatographic technology
- spectroscopic technology
1. Introduction
Food security has always been an issue of concern in the international community, and, in recent years, food contamination has become a major factor affecting food security. Contaminated food can not only adversely influence human health (poisoning events, chronic diseases, etc.) but also affect and slow down the economy. When people consume contaminated food, they need to spend a lot of money and time on treatment. There are many factors causing food contamination, such as biological, chemical, and physical factors. Among these, microbial contamination is common and mainly includes contamination by bacteria, fungi, molds, viruses, or their toxins and by-products [1][2]. Mycotoxins are common food contaminants, which can cause changes in the appearance, flavor, smell, and other characteristics of food [3][4][5][6][7]. Mycotoxins are secondary metabolites produced by fungi (e.g., Fusarium, Aspergillus, and Penicillium) that have multiple toxic effects on organisms and contaminate agricultural products (cereals, milk, etc.). More than 400 kinds of mycotoxins have been identified. Among them, aflatoxins (AFs) have become one of the major concerns due to their high toxicity and carcinogenicity, causing approximately 25% of animal deaths [8][9][10][11][12].
Edible vegetable oil plays an irreplaceable role in the human diet. The world oil crop output has increased year by year and had reached 635.5 million tons by 2021 [13]. From the growth of oil crops to the final product, i.e., oil, each link may be affected by external factors (such as mycotoxins), which may affect the quality and safety of edible vegetable oil [14]. This is because most oil crops, such as corn, peanut, soybean, rapeseed, sunflower seeds, olives, and nuts, are seasonal. During the growth process, they will be affected by climate, pests, and other factors and can be easily be infected by Aspergillus flavus. After harvest, the oil may deteriorate or be affected by mildew due to storage conditions (such as temperature and humidity, etc.) and storage methods [15]. At the same time, during the production of edible oil, fresh-pressed edible oil is vulnerable to contamination of raw materials infected with Aspergillus by aflatoxin B1 (AFB1) [16][17][18][19][20][21][22]. Therefore, contamination of edible vegetable oil products by AFB1 is a serious food safety problem (Figure 1) [20][23][24][25].
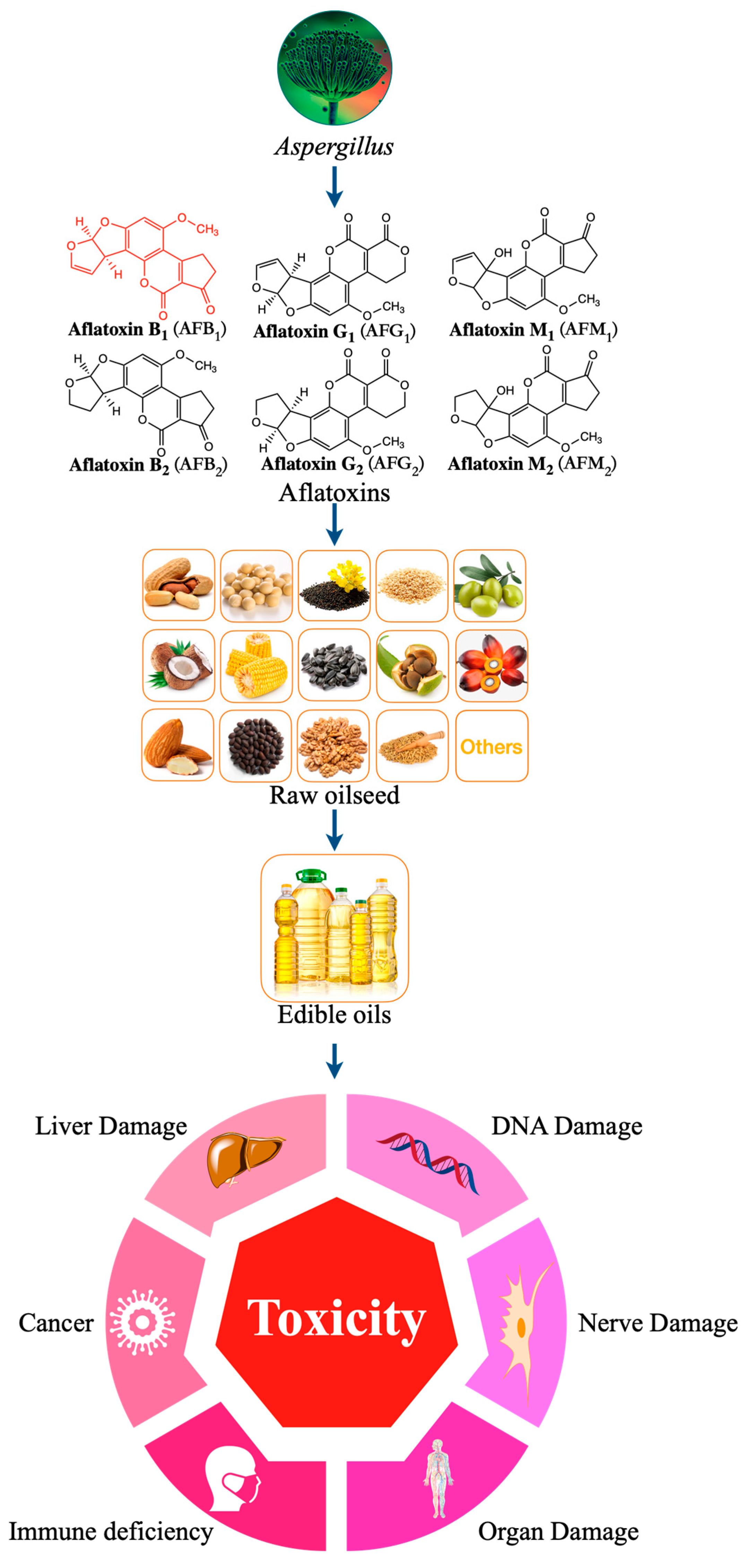
Figure 1. Harmful effects of different types aflatoxins contaminated edible oil.
The presence of aflatoxin is usually detected by using precision instruments, such as high-performance liquid chromatography–mass spectrometry (HPLC–MS), high-performance liquid chromatography–fluorescence detection (HPLC–FD), or other molecular techniques, while rapid detection is mainly realized by enzymatic immunoassay ELISA [26][27]. Although different methods are available for the detection of AFB1 toxicity, these methods require expensive equipment and complex sample pretreatment or can only be performed at relatively high concentrations [28]. Therefore, simple, sensitive, efficient, economical, rapid, and stable AFB1 detection methods are required. Recently, new technologies, such as biosensors, have been applied in many fields, such as health care and food detection. Because of their key advantages, such as convenient operation, rapid response, and excellent portability, these technologies can detect harmful substances in food sensitively and accurately, helping effectively avoid their harmful effects. They have attracted increasing attention of researchers and also promoted the rapid development of biosensors. With progress in nanotechnology, scientists are paying special attention to biosensors based on nanomaterials. These new biosensors or detection systems are sensitive, rapid, consistent, and cost-effective and can be used to detect AFB1 in food [29][30][31][32][33].
2. Importance of Aflatoxins
Aflatoxins are a type of mycotoxins. They are highly toxic metabolites of fungi, produced in food and agricultural products. They have severe toxic effects, such as immunosuppressive, nephrotoxic, teratogenic, carcinogenic, and mutagenic, on human and animal health [34][35][36][37][38].
Aflatoxins can be divided into aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2) according to their fluorescence properties and chromatographic mobility (Figure 1) [39][40][41]. Aflatoxin M1 (AFM1) and aflatoxin M2 (AFM2) are hydroxylated metabolites of AFB1 and AFB2, respectively. AFB1 is the most toxic among all AF species, with a high incidence rate and the most complex detection mechanism (Figure 2) [42].
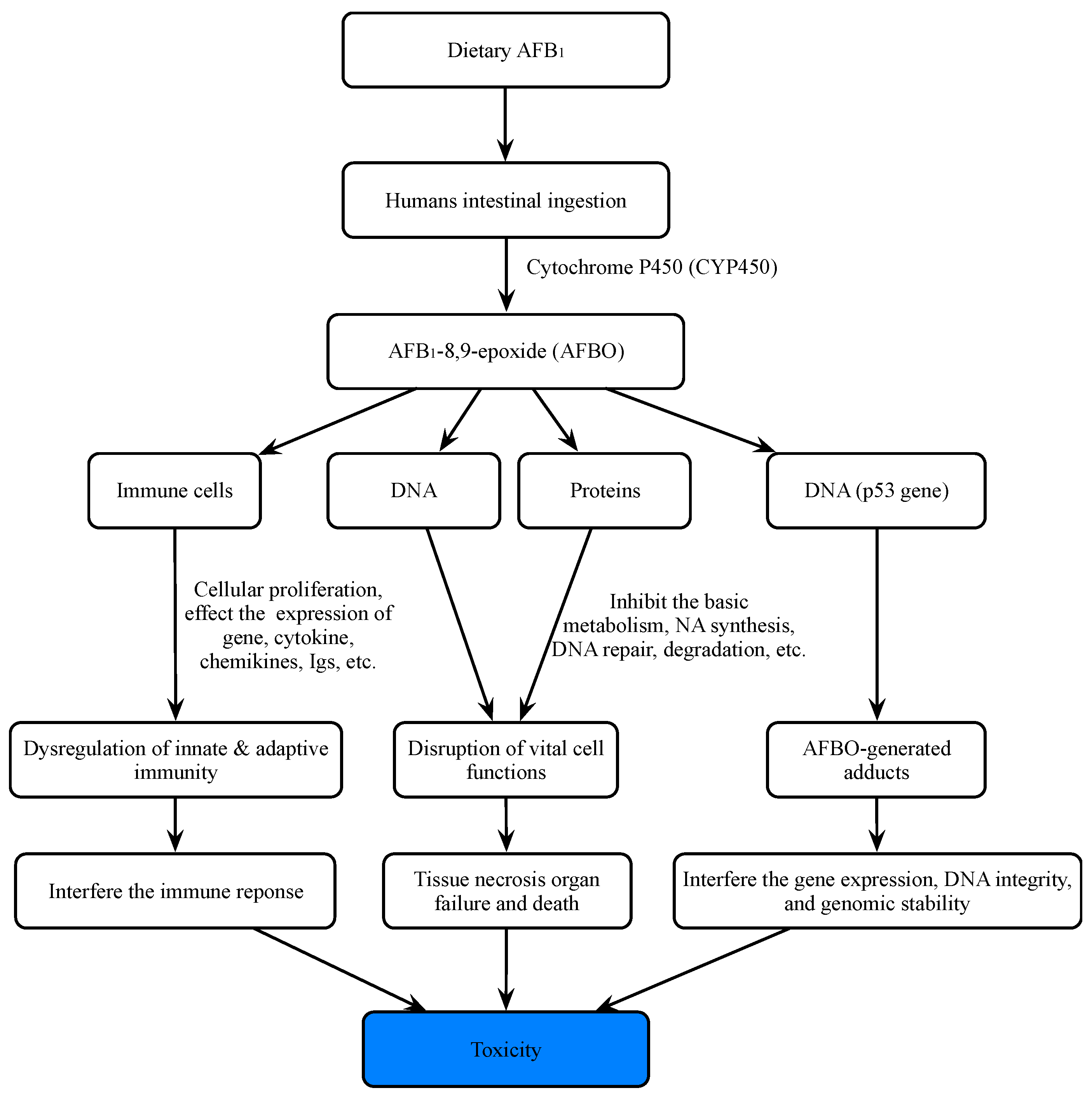
Figure 2. Main mechanisms of toxicity of aflatoxin B1 for humans.
AFB1 is a powerful carcinogenic, teratogenic, mutagenic, immunotoxic, hepatotoxic, and reproducible poison. Previous studies have shown that the toxicity of AFB1 is 10, 68, and 416 times that of KCN, arsenic and melamine, respectively [43][44] (Figure 2). Therefore, AFB1 has been classified as a class 1 carcinogen by many international authoritative organizations or institutions [45][46]. Due to the structural double bonds in the furan ring, AFB1 has high carcinogenicity and toxicity [17][47]. The lipophilic structure of atrial fibrillation promotes its entry into the blood through gastrointestinal and respiratory tracts [48][49]. Once AFB1 enters blood, it is distributed in various tissues and accumulates in the liver or other organs, resulting in liver cancer (Figure 3). In the liver, AFB1 produces a variety of metabolites through the hydroxylation and demethylation of the first-stage drug metabolism enzymes (for example, cytochrome P450 oxidase and CYP450 superfamily members, such as CYP1A2, CYP3A4, and CYP2A6) [50]. Metabolic reaction (internal and external) activates the final carcinogen AFB1 -8,9-epoxy metabolite, which covalently binds to cellular macromolecules (DNA, RNA, or protein) and plays a key role in acute and chronic poisoning. AFB1 residues also destroy the function of tumor suppressor genes (p53 and Rb) in the liver, which affects normal cells and leads to liver injury, increasing the probability of tumor and liver cirrhosis [51][52][53][54][55]. It is estimated that about 30% of liver cancers in the world are caused by AFB1. Its toxicity increases the infection rate of hepatitis B virus (HBV) and the risk of liver cancer [56]. A recent study found that the synergistic effect of AFB1 and HBV leads to liver cancer [50]. The reason is that HBV infection directly or indirectly exposes hepatocytes to AFB1 sensitive to tumors. The toxic effect of AFB1 is also related to dose, age, sex, nutrition, exposure time, and type [57]. In addition, AFB1 can be transmitted to the fetus through the placenta and affect the health of infants [58]. AFB1 exposure also inhibits immunity, thereby increasing the susceptibility to immunodeficiency virus attack and the probability of infection with other infectious diseases [59][60][61][62][63].
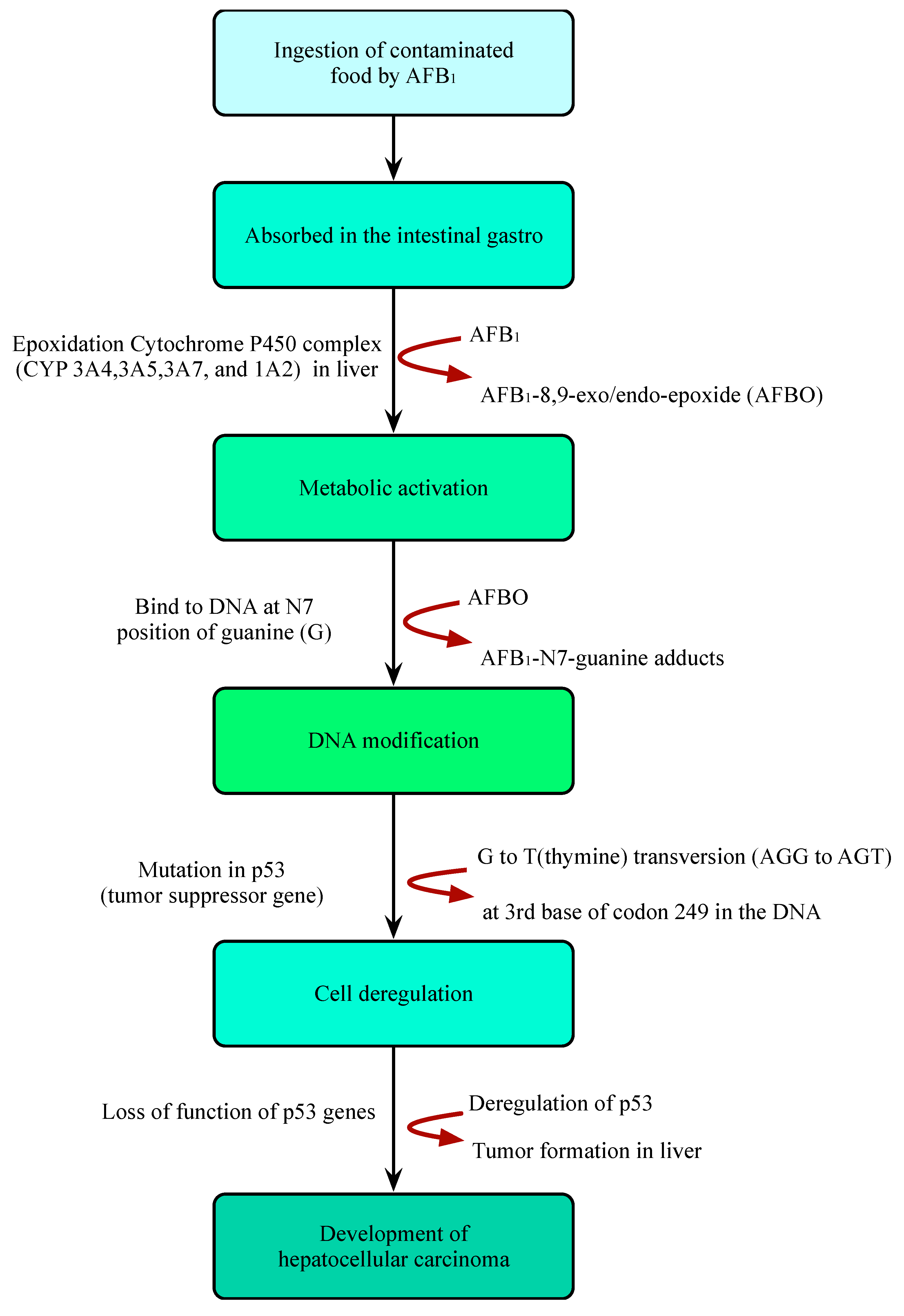
Figure 3. Illustration of the mechanism of hepatocellular carcinoma caused by ingestion of AFB1-contaminated foods.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196141
References
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307.
- Skrzydlewski, P.; Twaruzek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244.
- Holban, A.M.; Grumezescu, A.M. Microbial Contamination and Food Degradation; Academic Press: London, UK, 2018; Volume 10.
- Pickova, D.; Ostry, V.; Malir, F. A Recent Overview of Producers and Important Dietary Sources of Aflatoxins. Toxins 2021, 13, 186.
- Anater, A.; Manyes, L.; Meca, G.; Ferrer, E.; Luciano, F.B.; Pimpão, C.T.; Font, G. Mycotoxins and their consequences in aquaculture: A review. Aquaculture 2016, 451, 1–10.
- Wang, Y.; Nie, J.; Yan, Z.; Li, Z.; Cheng, Y.; Chang, W. Occurrence and co-occurrence of mycotoxins in nuts and dried fruits from China. Food Control 2018, 88, 181–189.
- Lee, H.J.; Ryu, D. Worldwide Occurrence of Mycotoxins in Cereals and Cereal-Derived Food Products: Public Health Perspectives of Their Co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051.
- Azam, M.S.; Ahmed, S.; Islam, M.N.; Maitra, P.; Islam, M.M.; Yu, D. Critical Assessment of Mycotoxins in Beverages and Their Control Measures. Toxins 2021, 13, 323.
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266.
- Ünüsan, N. Systematic review of mycotoxins in food and feeds in Turkey. Food Control 2019, 97, 1–14.
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food. Microbiol. 2015, 207, 87–102.
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609.
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022.
- Mao, X.; Yan, A.; Wan, Y.; Luo, D.; Yang, H. Dispersive Solid-Phase Extraction Using Microporous Sorbent UiO-66 Coupled to Gas Chromatography-Tandem Mass Spectrometry: A QuEChERS-Type Method for the Determination of Organophosphorus Pesticide Residues in Edible Vegetable Oils without Matrix Interference. J. Agric. Food Chem. 2019, 67, 1760–1770.
- Pitt, J.I.; Taniwaki, M.H.; Cole, M.B. Mycotoxin production in major crops as influenced by growing, harvesting, storage and processing, with emphasis on the achievement of Food Safety Objectives. Food Control 2013, 32, 205–215.
- Vasseghian, Y.; Moradi, M.; Dragoi, E.-N.; Khaneghah, A.M. A review on mycotoxins detection techniques in edible oils. Int. J. Environ. Anal. Chem. 2020, 102, 2125–2139.
- Javanmardi, F.; Khodaei, D.; Sheidaei, Z.; Bashiry, M.; Nayebzadeh, K.; Vasseghian, Y.; Mousavi Khaneghah, A. Decontamination of Aflatoxins in Edible Oils: A Comprehensive Review. Food Rev. Int. 2020, 38, 1–17.
- Shavakhi, F.; Rahmani, A.; Piravi-Vanak, Z. A global systematic review and meta-analysis on prevalence of the aflatoxin B1 contamination in olive oil. J. Food. Sci. Technol. 2022, 1–10.
- He, X.; Zhang, Y.; Yang, X.; Chen, M.; Pang, Y.; Shen, F.; Fang, Y.; Liu, Q.; Hu, Q. Estimating bulk optical properties of AFB1 contaminated edible oils in 300-900 nm by combining double integrating spheres technique with laser induced fluorescence spectroscopy. Food Chem. 2022, 375, 131666.
- Qi, N.; Yu, H.; Yang, C.; Gong, X.; Liu, Y.; Zhu, Y. Aflatoxin B1 in peanut oil from Western Guangdong, China, during 2016-2017. Food Addit. Contam. Part B 2019, 12, 45–51.
- Li, S.; Li, X.; Zhang, Q. Advances in the development of detection techniques for mycotoxins in vegetable oil. Chin. J. Chromatogr. 2019, 37, 569–580.
- Ji, J.; Jiang, M.; Zhang, Y.; Hou, J.; Sun, S. Co-occurrence of aflatoxins in plant oil products from China. Food Addit. Contam. Part B 2022, 1–8.
- Einolghozati, M.; Talebi-Ghane, E.; Ranjbar, A.; Mehri, F. Concentration of aflatoxins in edible vegetable oils: A systematic meta-analysis review. Eur. Food Res. Technol. 2021, 247, 2887–2897.
- Bordin, K.; Sawada, M.M.; Rodrigues, C.E.d.C.; da Fonseca, C.R.; Oliveira, C.A.F. Incidence of Aflatoxins in Oil Seeds and Possible Transfer to Oil: A Review. Food Eng. Rev. 2014, 6, 20–28.
- Shephard, G.S. Aflatoxins in peanut oil: Food safety concerns. World Mycotoxin J. 2018, 11, 149–158.
- Wu, L.X.; Ding, X.X.; Li, P.W.; Du, X.H.; Zhou, H.Y.; Bai, Y.Z.; Zhang, L.X. Aflatoxin contamination of peanuts at harvest in China from 2010 to 2013 and its relationship with climatic conditions. Food Control 2016, 60, 117–123.
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC, Trends Anal. Chem. 2019, 113, 140–156.
- Hayashi, Y.; Matsuda, R.; Maitani, T.; Imai, K.; Nishimura, W.; Ito, K.; Maeda, M. Precision, limit of detection and range of quantitation in competitive ELISA. Anal. Chem. 2004, 76, 1295–1301.
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8.
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109.
- Xue, Z.; Zhang, Y.; Yu, W.; Zhang, J.; Wang, J.; Wan, F.; Kim, Y.; Liu, Y.; Kou, X. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal. Chim. Acta. 2019, 1069, 1–27.
- Goud, K.Y.; Reddy, K.K.; Satyanarayana, M.; Kummari, S.; Gobi, K.V. A review on recent developments in optical and electrochemical aptamer-based assays for mycotoxins using advanced nanomaterials. Mikrochim. Acta 2019, 187, 29.
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. Gold nanobipyramids integrated ultrasensitive optical and electrochemical biosensor for Aflatoxin B1 detection. Talanta 2021, 222, 121578.
- Danesh, N.M.; Bostan, H.B.; Abnous, K.; Ramezani, M.; Youssefi, K.; Taghdisi, S.M.; Karimi, G. Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors: A review. TrAC Trends Anal. Chem. 2018, 99, 117–128.
- Castillo, G.; Poturnayová, A.; Šnejdárková, M.; Hianik, T.; Spinella, K.; Mosiello, L. Development of electrochemical aptasensor using dendrimers as an immobilization platform for detection of Aflatoxin B1 in food samples. In Proceedings of the 2015 XVIII AISEM Annual Conference, Trento, Italy, 3–5 February 2015; pp. 1–4.
- Zheng, M.Z.; Richard, J.L.; Binder, J. A review of rapid methods for the analysis of mycotoxins. Mycopathologia 2006, 161, 261–273.
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516.
- Robbins, C.A.; Swenson, L.J.; Nealley, M.L.; Gots, R.E.; Kelman, B.J. Health effects of mycotoxins in indoor air: A critical review. Appl. Occup. Environ. Hyg. 2000, 15, 773–784.
- Catanante, G.; Rhouati, A.; Hayat, A.; Marty, J.L. An Overview of Recent Electrochemical Immunosensing Strategies for Mycotoxins Detection. Electroanalysis 2016, 28, 1750–1763.
- Yao, H.; Hruska, Z.; Di Mavungu, J.D. Developments in detection and determination of aflatoxins. World Mycotoxin J. 2015, 8, 181–191.
- Liu, D.; Li, W.; Zhu, C.; Li, Y.; Shen, X.; Li, L.; Yan, X.; You, T. Recent progress on electrochemical biosensing of aflatoxins: A review. TrAC Trends Anal. Chem. 2020, 133, 115966.
- Hui, Y.; Wang, B.; Ren, R.; Zhao, A.; Zhang, F.; Song, S.; He, Y. An electrochemical aptasensor based on DNA-AuNPs-HRP nanoprobes and exonuclease-assisted signal amplification for detection of aflatoxin B1. Food Control 2020, 109, 106902.
- Luan, Y.; Chen, Z.; Xie, G.; Chen, J.; Lu, A.; Li, C.; Fu, H.; Ma, Z.; Wang, J. Rapid Visual Detection of Aflatoxin B1 by Label-Free Aptasensor Using Unmodified Gold Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 1357–1361.
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2229–2234.
- Taghdisi, S.M.; Danesh, N.M.; Ramezani, M.; Abnous, K. A new amplified fluorescent aptasensor based on hairpin structure of G-quadruplex oligonucleotide-Aptamer chimera and silica nanoparticles for sensitive detection of aflatoxin B1 in the grape juice. Food Chem. 2018, 268, 342–346.
- Henry, S.H.; Bosch, F.X.; Troxell, T.C.; Bolger, P.M. Reducing liver cancer--global control of aflatoxin. Science 1999, 286, 2453–2454.
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A Structure Identification and Toxicity Assessment of the Degradation Products of Aflatoxin B(1) in Peanut Oil under UV Irradiation. Toxins 2016, 8, 332.
- Mousavi Khaneghah, A.; Eş, I.; Raeisi, S.; Fakhri, Y. Aflatoxins in cereals: State of the art. J. Food Saf. 2018, 38, 12532.
- Qureshi, H. Is Aflatoxin B1 A Biomarker for Pathogenic Potential of Aspergillus flavus? J. Cell Sci. 2014, 5, 1000188.
- Ali, N. Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197.
- Sun, C.; Liao, X.; Jia, B.; Shi, L.; Zhang, D.; Wang, R.; Zhou, L.; Kong, W. Development of a quantum dots-based label-free electrochemiluminescence immunosensor for sensitive determination of aflatoxin B1 in lotus seed. Mikrochim. Acta 2020, 187, 236.
- Bhardwaj, H.; Sumana, G.; Marquette, C.A. A label-free ultrasensitive microfluidic surface Plasmon resonance biosensor for Aflatoxin B1 detection using nanoparticles integrated gold chip. Food Chem. 2020, 307, 125530.
- Ramalho, L.N.Z.; Porta, L.D.; Rosim, R.E.; Petta, T.; Augusto, M.J.; Silva, D.M.; Ramalho, F.S.; Oliveira, C.A.F. Aflatoxin B1 residues in human livers and their relationship with markers of hepatic carcinogenesis in Sao Paulo, Brazil. Toxicol. Rep. 2018, 5, 777–784.
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120 (Suppl. 1), S28–S48.
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100.
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Liu, J.; Wang, L.Y.; Lu, S.N.; Lee, M.H.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int. J. Cancer 2017, 141, 711–720.
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237.
- Wild, C.P.; Turner, P.C. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481.
- Zhou, Q.; Tang, D. Recent advances in photoelectrochemical biosensors for analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2020, 124, 115814.
- Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances in Nanomaterial-mediated Bio and immune sensors for detection of aflatoxin in food products. TrAC Trends Anal. Chem. 2017, 87, 112–128.
- Keenan, J.; Jolly, P.; Preko, P.; Baidoo, J.; Wang, J.S.; Phillips, T.D.; Williams, J.H.; McGwin, G., Jr. Association Between Aflatoxin B1 Albumin Adduct Levels and Tuberculosis Infection Among HIV+ Ghanaians. Arch. Clin. Microbiol. 2011, 2, 3.
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983.
- Gong, Y.; Hounsa, A.; Egal, S.; Turner, P.C.; Sutcliffe, A.E.; Hall, A.J.; Cardwell, K.; Wild, C.P. Postweaning exposure to aflatoxin results in impaired child growth: A longitudinal study in Benin, West Africa. Environ. Health Persp. 2004, 112, 1334–1338.
This entry is offline, you can click here to edit this entry!
