Diabetes mellitus type 2 (also known as type 2 diabetes) is a long-term metabolic disorder that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urination, and unexplained weight loss. Symptoms may also include increased hunger, feeling tired, and sores that do not heal. Often symptoms come on slowly. Long-term complications from high blood sugar include heart disease, strokes, diabetic retinopathy which can result in blindness, kidney failure, and poor blood flow in the limbs which may lead to amputations. The sudden onset of hyperosmolar hyperglycemic state may occur; however, ketoacidosis is uncommon. Type 2 diabetes primarily occurs as a result of obesity and lack of exercise. Some people are more genetically at risk than others. Type 2 diabetes makes up about 90% of cases of diabetes, with the other 10% due primarily to diabetes mellitus type 1 and gestational diabetes. In diabetes mellitus type 1 there is a lower total level of insulin to control blood glucose, due to an autoimmune induced loss of insulin-producing beta cells in the pancreas. Diagnosis of diabetes is by blood tests such as fasting plasma glucose, oral glucose tolerance test, or glycated hemoglobin (A1C). Type 2 diabetes is partly preventable by staying a normal weight, exercising regularly, and eating properly. Treatment involves exercise and dietary changes. If blood sugar levels are not adequately lowered, the medication metformin is typically recommended. Many people may eventually also require insulin injections. In those on insulin, routinely checking blood sugar levels is advised; however, this may not be needed in those taking pills. Bariatric surgery often improves diabetes in those who are obese. Rates of type 2 diabetes have increased markedly since 1960 in parallel with obesity. As of 2015 there were approximately 392 million people diagnosed with the disease compared to around 30 million in 1985. Typically it begins in middle or older age, although rates of type 2 diabetes are increasing in young people. Type 2 diabetes is associated with a ten-year-shorter life expectancy. Diabetes was one of the first diseases described. The importance of insulin in the disease was determined in the 1920s.
- oral glucose tolerance
- diabetes mellitus type 1
- glycated hemoglobin
1. Signs and Symptoms
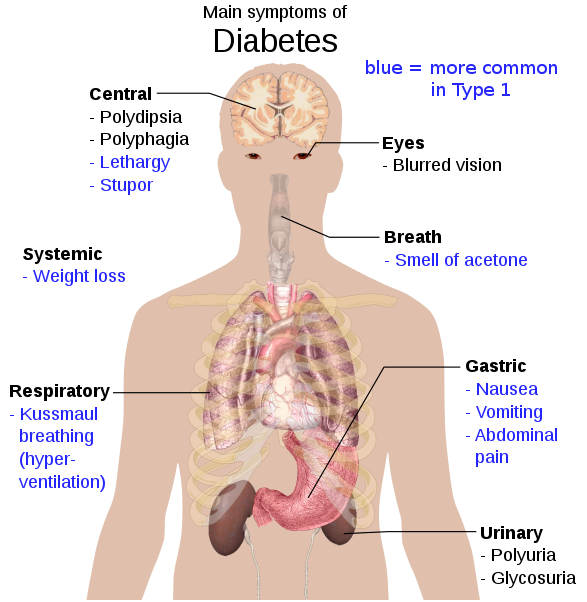
The classic symptoms of diabetes are polyuria (frequent urination), polydipsia (increased thirst), polyphagia (increased hunger), and weight loss.[1] Other symptoms that are commonly present at diagnosis include a history of blurred vision, itchiness, peripheral neuropathy, recurrent vaginal infections, and fatigue.[2] Many people, however, have no symptoms during the first few years and are diagnosed on routine testing.[2] A small number of people with type 2 diabetes mellitus can develop a hyperosmolar hyperglycemic state (a condition of very high blood sugar associated with a decreased level of consciousness and low blood pressure).[2]
1.1. Complications
Type 2 diabetes is typically a chronic disease associated with a ten-year-shorter life expectancy.[3] This is partly due to a number of complications with which it is associated, including: two to four times the risk of cardiovascular disease, including ischemic heart disease and stroke; a 20-fold increase in lower limb amputations, and increased rates of hospitalizations.[3] In the developed world, and increasingly elsewhere, type 2 diabetes is the largest cause of nontraumatic blindness and kidney failure.[4] It has also been associated with an increased risk of cognitive dysfunction and dementia through disease processes such as Alzheimer's disease and vascular dementia.[5] Other complications include acanthosis nigricans, sexual dysfunction, and frequent infections.[1]
2. Cause
The development of type 2 diabetes is caused by a combination of lifestyle and genetic factors.[4][6] While some of these factors are under personal control, such as diet and obesity, other factors are not, such as increasing age, female gender, and genetics.[3] A lack of sleep has been linked to type 2 diabetes.[7] This is believed to act through its effect on metabolism.[7] The nutritional status of a mother during fetal development may also play a role, with one proposed mechanism being that of altered DNA methylation.[8] The intestinal bacteria Prevotella copri and Bacteroides vulgatus have been connected with type 2 diabetes.[9]
2.1. Lifestyle
Lifestyle factors are important to the development of type 2 diabetes, including obesity and being overweight (defined by a body mass index of greater than 25), lack of physical activity, poor diet, stress, and urbanization.[3][10] Excess body fat is associated with 30% of cases in those of Chinese and Japanese descent, 60–80% of cases in those of European and African descent, and 100% of cases in Pima Indians and Pacific Islanders.[2] Among those who are not obese, a high waist–hip ratio is often present.[2] Smoking appears to increase the risk of type 2 diabetes mellitus.[11]
Dietary factors also influence the risk of developing type 2 diabetes. Consumption of sugar-sweetened drinks in excess is associated with an increased risk.[12][13] The type of fats in the diet are important, with saturated fats and trans fatty acids increasing the risk, and polyunsaturated and monounsaturated fat decreasing the risk.[6] Eating a lot of white rice appears to play a role in increasing risk.[14] A lack of exercise is believed to cause 7% of cases.[15] Persistent organic pollutants may play a role.[16]
2.2. Genetics
Most cases of diabetes involve many genes, with each being a small contributor to an increased probability of becoming a type 2 diabetic.[3] If one identical twin has diabetes, the chance of the other developing diabetes within his lifetime is greater than 90%, while the rate for nonidentical siblings is 25–50%.[2] As of 2011, more than 36 genes had been found that contribute to the risk of type 2 diabetes.[17] All of these genes together still only account for 10% of the total heritable component of the disease.[17] The TCF7L2 allele, for example, increases the risk of developing diabetes by 1.5 times and is the greatest risk of the common genetic variants.[2] Most of the genes linked to diabetes are involved in beta cell functions.[2]
There are a number of rare cases of diabetes that arise due to an abnormality in a single gene (known as monogenic forms of diabetes or "other specific types of diabetes").[2][3] These include maturity onset diabetes of the young (MODY), Donohue syndrome, and Rabson–Mendenhall syndrome, among others.[3] Maturity onset diabetes of the young constitute 1–5% of all cases of diabetes in young people.[18]
2.3. Medical conditions
There are a number of medications and other health problems that can predispose to diabetes.[19] Some of the medications include: glucocorticoids, thiazides, beta blockers, atypical antipsychotics,[20] and statins.[21] Those who have previously had gestational diabetes are at a higher risk of developing type 2 diabetes.[1] Other health problems that are associated include: acromegaly, Cushing's syndrome, hyperthyroidism, pheochromocytoma, and certain cancers such as glucagonomas.[19] Testosterone deficiency is also associated with type 2 diabetes.[22][23]
3. Pathophysiology
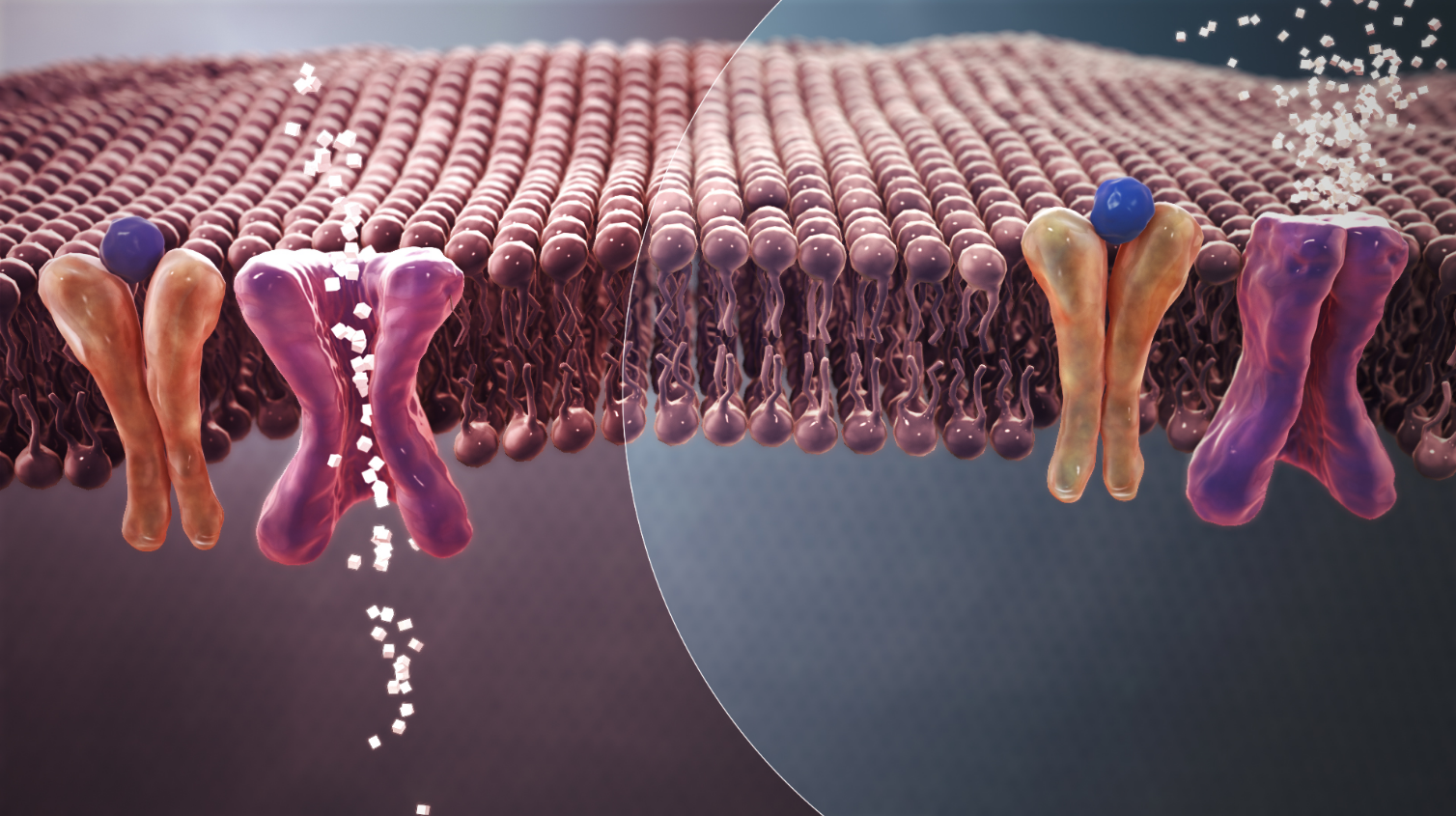
Type 2 diabetes is due to insufficient insulin production from beta cells in the setting of insulin resistance.[2] Insulin resistance, which is the inability of cells to respond adequately to normal levels of insulin, occurs primarily within the muscles, liver, and fat tissue.[24] In the liver, insulin normally suppresses glucose release. However, in the setting of insulin resistance, the liver inappropriately releases glucose into the blood.[3] The proportion of insulin resistance versus beta cell dysfunction differs among individuals, with some having primarily insulin resistance and only a minor defect in insulin secretion and others with slight insulin resistance and primarily a lack of insulin secretion.[2]
Other potentially important mechanisms associated with type 2 diabetes and insulin resistance include: increased breakdown of lipids within fat cells, resistance to and lack of incretin, high glucagon levels in the blood, increased retention of salt and water by the kidneys, and inappropriate regulation of metabolism by the central nervous system.[3] However, not all people with insulin resistance develop diabetes, since an impairment of insulin secretion by pancreatic beta cells is also required.[2]
4. Diagnosis
The World Health Organization definition of diabetes (both type 1 and type 2) is for a single raised glucose reading with symptoms, otherwise raised values on two occasions, of either:[25]
- fasting plasma glucose ≥ 7.0 mmol/l (126 mg/dl)
- or
- with a glucose tolerance test, two hours after the oral dose a plasma glucose ≥ 11.1 mmol/l (200 mg/dl)
A random blood sugar of greater than 11.1 mmol/l (200 mg/dl) in association with typical symptoms[1] or a glycated hemoglobin (HbA1c) of ≥ 48 mmol/mol (≥ 6.5 DCCT %) is another method of diagnosing diabetes.[3] In 2009 an International Expert Committee that included representatives of the American Diabetes Association (ADA), the International Diabetes Federation (IDF), and the European Association for the Study of Diabetes (EASD) recommended that a threshold of ≥ 48 mmol/mol (≥ 6.5 DCCT %) should be used to diagnose diabetes.[26] This recommendation was adopted by the American Diabetes Association in 2010.[27] Positive tests should be repeated unless the person presents with typical symptoms and blood sugars >11.1 mmol/l (>200 mg/dl).[26]
Threshold for diagnosis of diabetes is based on the relationship between results of glucose tolerance tests, fasting glucose or HbA1c and complications such as retinal problems.[3] A fasting or random blood sugar is preferred over the glucose tolerance test, as they are more convenient for people.[3] HbA1c has the advantages that fasting is not required and results are more stable but has the disadvantage that the test is more costly than measurement of blood glucose.[28] It is estimated that 20% of people with diabetes in the United States do not realize that they have the disease.[3]
Diabetes mellitus type 2 is characterized by high blood glucose in the context of insulin resistance and relative insulin deficiency.[29] This is in contrast to diabetes mellitus type 1 in which there is an absolute insulin deficiency due to destruction of islet cells in the pancreas and gestational diabetes mellitus that is a new onset of high blood sugars associated with pregnancy.[2] Type 1 and type 2 diabetes can typically be distinguished based on the presenting circumstances.[26] If the diagnosis is in doubt antibody testing may be useful to confirm type 1 diabetes and C-peptide levels may be useful to confirm type 2 diabetes,[30] with C-peptide levels normal or high in type 2 diabetes, but low in type 1 diabetes.[31]
5. Screening
No major organization recommends universal screening for diabetes as there is no evidence that such a program improve outcomes.[32][33] Screening is recommended by the United States Preventive Services Task Force (USPSTF) in adults without symptoms whose blood pressure is greater than 135/80 mmHg.[34] For those whose blood pressure is less, the evidence is insufficient to recommend for or against screening.[34] There is no evidence that it changes the risk of death in this group of people.[33] They also recommend screening among those who are overweight and between the ages of 40 and 70.[35]
The World Health Organization recommends testing those groups at high risk[32] and in 2014 the USPSTF is considering a similar recommendation.[36] High-risk groups in the United States include: those over 45 years old; those with a first degree relative with diabetes; some ethnic groups, including Hispanics, African-Americans, and Native-Americans; a history of gestational diabetes; polycystic ovary syndrome; excess weight; and conditions associated with metabolic syndrome.[1] The American Diabetes Association recommends screening those who have a BMI over 25 (in people of Asian descent screening is recommended for a BMI over 23).[37]
6. Prevention
Onset of type 2 diabetes can be delayed or prevented through proper nutrition and regular exercise.[38][39] Intensive lifestyle measures may reduce the risk by over half.[4][40] The benefit of exercise occurs regardless of the person's initial weight or subsequent weight loss.[41] High levels of physical activity reduce the risk of diabetes by about 28%.[42] Evidence for the benefit of dietary changes alone, however, is limited,[43] with some evidence for a diet high in green leafy vegetables[44] and some for limiting the intake of sugary drinks.[12] In those with impaired glucose tolerance, diet and exercise either alone or in combination with metformin or acarbose may decrease the risk of developing diabetes.[4][45] Lifestyle interventions are more effective than metformin.[4] A 2017 review found that, long term, lifestyle changes decreased the risk by 28%, while medication does not reduce risk after withdrawal.[46] While low vitamin D levels are associated with an increased risk of diabetes, correcting the levels by supplementing vitamin D3 does not improve that risk.[47]
7. Management
Management of type 2 diabetes focuses on lifestyle interventions, lowering other cardiovascular risk factors, and maintaining blood glucose levels in the normal range.[4] Self-monitoring of blood glucose for people with newly diagnosed type 2 diabetes may be used in combination with education,[48] however the benefit of self monitoring in those not using multi-dose insulin is questionable.[4][49] In those who do not want to measure blood levels, measuring urine levels may be done.[48] Managing other cardiovascular risk factors, such as hypertension, high cholesterol, and microalbuminuria, improves a person's life expectancy.[4] Decreasing the systolic blood pressure to less than 140 mmHg is associated with a lower risk of death and better outcomes.[50] Intensive blood pressure management (less than 130/80 mmHg) as opposed to standard blood pressure management (less than 140-160 mmHg systolic to 85–100 mmHg diastolic) results in a slight decrease in stroke risk but no effect on overall risk of death.[51]
Intensive blood sugar lowering (HbA1c<6%) as opposed to standard blood sugar lowering (HbA1c of 7–7.9%) does not appear to change mortality.[52][53] The goal of treatment is typically an HbA1c of 7 to 8% or a fasting glucose of less than 7.2 mmol/L (130 mg/dl); however these goals may be changed after professional clinical consultation, taking into account particular risks of hypoglycemia and life expectancy.[37][54][55] Despite guidelines recommending that intensive blood sugar control be based on balancing immediate harms with long-term benefits, many people – for example people with a life expectancy of less than nine years who will not benefit, are over-treated.[56]
It is recommended that all people with type 2 diabetes get regular eye examination.[2] There is weak evidence suggesting that treating gum disease by scaling and root planing may result in a small short-term improvement in blood sugar levels for people with diabetes.[57] There is no evidence to suggest that this improvement in blood sugar levels is maintained longer than 4 months.[57] There is also not enough evidence to determine if medications to treat gum disease are effective at lowering blood sugar levels.[57]
7.1. Lifestyle
A proper diet and exercise are the foundations of diabetic care,[1] with a greater amount of exercise yielding better results.[58] Exercise improves blood sugar control, decreases body fat content and decreases blood lipid levels, and these effects are evident even without weight loss.[59] Aerobic exercise leads to a decrease in HbA1c and improved insulin sensitivity.[60] Resistance training is also useful and the combination of both types of exercise may be most effective.[60]
A diabetic diet that promotes weight loss is important.[61] While the best diet type to achieve this is controversial,[61] a low glycemic index diet or low carbohydrate diet has been found to improve blood sugar control.[62][63] A very low calorie diet, begun shortly after the onset of type 2 diabetes, can result in remission of the condition.[64]
Vegetarian diets in general have been related to lower diabetes risk, but do not offer advantages compared with diets which allow moderate amounts of animal products.[65] There is not enough evidence to suggest that cinnamon improves blood sugar levels in people with type 2 diabetes.[66]
Culturally appropriate education may help people with type 2 diabetes control their blood sugar levels, for up to 24 months.[67] If changes in lifestyle in those with mild diabetes has not resulted in improved blood sugars within six weeks, medications should then be considered.[1] There is not enough evidence to determine if lifestyle interventions affect mortality in those who already have DM2.[40]
7.2. Medications
There are several classes of anti-diabetic medications available. Metformin is generally recommended as a first line treatment as there is some evidence that it decreases mortality;[4][68][69] however, this conclusion is questioned.[70] Metformin should not be used in those with severe kidney or liver problems.[1]
A second oral agent of another class or insulin may be added if metformin is not sufficient after three months.[54] Other classes of medications include: sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, SGLT2 inhibitors, and glucagon-like peptide-1 analogs.[54] As of 2015 there was no significant difference between these agents.[54] A 2018 review found that SGLT2 inhibitors may be better than glucagon-like peptide-1 analogs or dipeptidyl peptidase-4 inhibitors.[71]
Rosiglitazone, a thiazolidinedione, has not been found to improve long-term outcomes even though it improves blood sugar levels.[72] Additionally it is associated with increased rates of heart disease and death.[73] Angiotensin-converting enzyme inhibitors (ACEIs) prevent kidney disease and improve outcomes in those with diabetes.[74][75] The similar medications angiotensin receptor blockers (ARBs) do not.[75] A 2016 review recommended treating to a systolic blood pressure of 140 to 150 mmHg.[76]
Injections of insulin may either be added to oral medication or used alone.[4] Most people do not initially need insulin.[2] When it is used, a long-acting formulation is typically added at night, with oral medications being continued.[1][4] Doses are then increased to effect (blood sugar levels being well controlled).[4] When nightly insulin is insufficient, twice daily insulin may achieve better control.[1] The long acting insulins glargine and detemir are equally safe and effective,[77] and do not appear much better than neutral protamine Hagedorn (NPH) insulin, but as they are significantly more expensive, they are not cost effective as of 2010.[78] In those who are pregnant insulin is generally the treatment of choice.[1]
Vitamin D supplementation to people with type 2 diabetes may improve markers of insulin resistance and HbA1c.[79]
7.3. Surgery
Weight loss surgery in those who are obese is an effective measure to treat diabetes.[80] Many are able to maintain normal blood sugar levels with little or no medication following surgery[81] and long-term mortality is decreased.[82] There however is some short-term mortality risk of less than 1% from the surgery.[83] The body mass index cutoffs for when surgery is appropriate are not yet clear.[82] It is recommended that this option be considered in those who are unable to get both their weight and blood sugar under control.[84][85]
8. Epidemiology
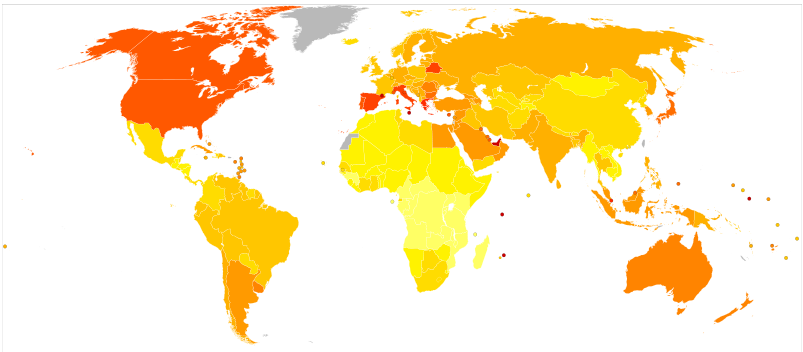
Globally as of 2015 it was estimated that there were 392 million people with type 2 diabetes making up about 90% of diabetes cases.[3][86] This is equivalent to about 6% of the world's population.[86] Diabetes is common both in the developed and the developing world.[3] It remains uncommon, however, in the underdeveloped world.[2]
Women seem to be at a greater risk as do certain ethnic groups,[3][87] such as South Asians, Pacific Islanders, Latinos, and Native Americans.[1] This may be due to enhanced sensitivity to a Western lifestyle in certain ethnic groups.[88] Traditionally considered a disease of adults, type 2 diabetes is increasingly diagnosed in children in parallel with rising obesity rates.[3] Type 2 diabetes is now diagnosed as frequently as type 1 diabetes in teenagers in the United States.[2]
Rates of diabetes in 1985 were estimated at 30 million, increasing to 135 million in 1995 and 217 million in 2005.[89] This increase is believed to be primarily due to the global population aging, a decrease in exercise, and increasing rates of obesity.[89] The five countries with the greatest number of people with diabetes as of 2000 are India having 31.7 million, China 20.8 million, the United States 17.7 million, Indonesia 8.4 million, and Japan 6.8 million.[90] It is recognized as a global epidemic by the World Health Organization.[91]
9. History
Diabetes is one of the first diseases described[92] with an Egyptian manuscript from c. 1500 BCE mentioning "too great emptying of the urine."[93] The first described cases are believed to be of type 1 diabetes.[93] Indian physicians around the same time identified the disease and classified it as madhumeha or honey urine noting that the urine would attract ants.[93] The term "diabetes" or "to pass through" was first used in 230 BCE by the Greek Apollonius Of Memphis.[93] The disease was rare during the time of the Roman empire with Galen commenting that he had only seen two cases during his career.[93]
Type 1 and type 2 diabetes were identified as separate conditions for the first time by the Indian physicians Sushruta and Charaka in 400–500 AD with type 1 associated with youth and type 2 with being overweight.[93] The term "mellitus" or "from honey" was added by the Briton John Rolle in the late 1700s to separate the condition from diabetes insipidus which is also associated with frequent urination.[93] Effective treatment was not developed until the early part of the 20th century when the Canadians Frederick Banting and Charles Best discovered insulin in 1921 and 1922.[93] This was followed by the development of the long acting NPH insulin in the 1940s.[93]
The content is sourced from: https://handwiki.org/wiki/Medicine:Diabetes_mellitus_type_2
References
- Vijan, S (2010-03-02). "Type 2 diabetes". Annals of Internal Medicine 152 (5): ITC31–ITC15; quiz ITC316. doi:10.7326/0003-4819-152-5-201003020-01003. PMID 20194231. https://dx.doi.org/10.7326%2F0003-4819-152-5-201003020-01003
- Gardner, David G.; Shoback, Dolores, eds (2011). "Chapter 17: Pancreatic hormones & diabetes mellitus". Greenspan's basic & clinical endocrinology (9th ed.). New York: McGraw-Hill Medical. ISBN 0-07-162243-8. OCLC 613429053. http://www.worldcat.org/oclc/613429053
- Melmed, Shlomo; Polonsky, Kenneth S.; Larsen, P. Reed et al., eds. Williams textbook of endocrinology. (12th ed.). Philadelphia: Elsevier/Saunders. pp. 1371–1435. ISBN 978-1-4377-0324-5.
- "Management of blood glucose in type 2 diabetes mellitus". American Family Physician 79 (1): 29–36. January 2009. PMID 19145963. http://www.ncbi.nlm.nih.gov/pubmed/19145963
- Pasquier, F (October 2010). "Diabetes and cognitive impairment: how to evaluate the cognitive status?". Diabetes & Metabolism 36 Suppl 3: S100–05. doi:10.1016/S1262-3636(10)70475-4. PMID 21211730. https://dx.doi.org/10.1016%2FS1262-3636%2810%2970475-4
- Risérus, U; Willett, WC; Hu, FB (January 2009). "Dietary fats and prevention of type 2 diabetes". Progress in Lipid Research 48 (1): 44–51. doi:10.1016/j.plipres.2008.10.002. PMID 19032965. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2654180
- Touma, C; Pannain, S (August 2011). "Does lack of sleep cause diabetes?". Cleveland Clinic Journal of Medicine 78 (8): 549–58. doi:10.3949/ccjm.78a.10165. PMID 21807927. https://dx.doi.org/10.3949%2Fccjm.78a.10165
- Christian, P; Stewart, CP (March 2010). "Maternal micronutrient deficiency, fetal development, and the risk of chronic disease". The Journal of Nutrition 140 (3): 437–45. doi:10.3945/jn.109.116327. PMID 20071652. https://dx.doi.org/10.3945%2Fjn.109.116327
- Pedersen, HK; Gudmundsdottir, V; Nielsen, HB et al. (21 July 2016). "Human gut microbes impact host serum metabolome and insulin sensitivity". Nature 535 (7612): 376–81. doi:10.1038/nature18646. PMID 27409811. Bibcode: 2016Natur.535..376P. https://dx.doi.org/10.1038%2Fnature18646
- Abdullah, A; Peeters, A; de Courten, M; Stoelwinder, J (September 2010). "The magnitude of association between overweight and obesity and the risk of diabetes: a meta-analysis of prospective cohort studies.". Diabetes Research and Clinical Practice 89 (3): 309–19. doi:10.1016/j.diabres.2010.04.012. PMID 20493574. https://dx.doi.org/10.1016%2Fj.diabres.2010.04.012
- Pan, A; Wang, Y; Talaei, M; Hu, FB; Wu, T (17 September 2015). "Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis". The Lancet Diabetes & Endocrinology 3 (12): 958–67. doi:10.1016/S2213-8587(15)00316-2. PMID 26388413. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4656094
- Malik, VS; Popkin, BM; Bray, GA; Després, JP; Hu, FB (2010-03-23). "Sugar Sweetened Beverages, Obesity, Type 2 Diabetes and Cardiovascular Disease risk". Circulation 121 (11): 1356–64. doi:10.1161/CIRCULATIONAHA.109.876185. PMID 20308626. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2862465
- Malik, VS; Popkin, BM; Bray, GA; Després, JP; Willett, WC; Hu, FB (November 2010). "Sugar-Sweetened Beverages and Risk of Metabolic Syndrome and Type 2 Diabetes: A meta-analysis". Diabetes Care 33 (11): 2477–83. doi:10.2337/dc10-1079. PMID 20693348. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2963518
- Hu, EA; Pan, A; Malik, V; Sun, Q (2012-03-15). "White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review". The BMJ 344: e1454. doi:10.1136/bmj.e1454. PMID 22422870. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3307808
- Lee, I-Min; Shiroma, Eric J; Lobelo, Felipe; Puska, Pekka; Blair, Steven N; Katzmarzyk, Peter T (1 July 2012). "Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy". The Lancet 380 (9838): 219–29. doi:10.1016/S0140-6736(12)61031-9. PMID 22818936. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3645500
- Lind, L; Lind, PM (Jun 2012). "Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease?". Journal of Internal Medicine 271 (6): 537–53. doi:10.1111/j.1365-2796.2012.02536.x. PMID 22372998. https://dx.doi.org/10.1111%2Fj.1365-2796.2012.02536.x
- Herder, C; Roden, M (June 2011). "Genetics of type 2 diabetes: pathophysiologic and clinical relevance". European Journal of Clinical Investigation 41 (6): 679–92. doi:10.1111/j.1365-2362.2010.02454.x. PMID 21198561. https://dx.doi.org/10.1111%2Fj.1365-2362.2010.02454.x
- "Monogenic Forms of Diabetes: Neonatal Diabetes Mellitus and Maturity-onset Diabetes of the Young". National Diabetes Information Clearinghouse (NDIC) (National Institute of Diabetes and Digestive and Kidney Diseases, NIH). March 2007. Archived from the original on 2008-07-04. https://web.archive.org/web/20080704103703/http://diabetes.niddk.nih.gov/dm/pubs/mody/. Retrieved 2008-08-04.
- Funnell, Martha M.; Anderson, Robert M. (2008). "Influencing self-management: from compliance to collaboration". in Feinglos, Mark N.; Bethel, M. Angelyn. Type 2 diabetes mellitus: an evidence-based approach to practical management. Contemporary endocrinology. Totowa, NJ: Humana Press. p. 462. ISBN 978-1-58829-794-5. OCLC 261324723. https://books.google.com/books?id=Cn-9BAAAQBAJ&pg=PA455.
- Izzedine, H; Launay-Vacher, V; Deybach, C; Bourry, E; Barrou, B; Deray, G (November 2005). "Drug-induced diabetes mellitus". Expert Opinion on Drug Safety 4 (6): 1097–1109. doi:10.1517/14740338.4.6.1097. PMID 16255667. https://dx.doi.org/10.1517%2F14740338.4.6.1097
- Sampson, UK; Linton, MF; Fazio, S (July 2011). "Are statins diabetogenic?". Current Opinion in Cardiology 26 (4): 342–47. doi:10.1097/HCO.0b013e3283470359. PMID 21499090. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3341610
- Saad, Farid; Gooren, Louis (March 2009). "The role of testosterone in the metabolic syndrome: a review". The Journal of Steroid Biochemistry and Molecular Biology 114 (1–2): 40–43. doi:10.1016/j.jsbmb.2008.12.022. PMID 19444934. https://dx.doi.org/10.1016%2Fj.jsbmb.2008.12.022
- Farrell, JoAnne B.; Deshmukh, Anjali; Baghaie, Ali A. (2008). "Low testosterone and the association with type 2 diabetes". The Diabetes Educator 34 (5): 799–806. doi:10.1177/0145721708323100. PMID 18832284. https://dx.doi.org/10.1177%2F0145721708323100
- Diabetes mellitus a guide to patient care.. Philadelphia: Lippincott Williams & Wilkins. 2007. p. 15. ISBN 978-1-58255-732-8. https://books.google.com/books?id=fiAclxvKblkC&pg=PA15.
- World Health Organization. "Definition, diagnosis and classification of diabetes mellitus and its complications: Report of a WHO Consultation. Part 1. Diagnosis and classification of diabetes mellitus". Archived from the original on 2007-05-29. https://web.archive.org/web/20070529092627/http://www.who.int/diabetes/publications/en/. Retrieved 2007-05-29.
- International Expert, Committee (July 2009). "International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes". Diabetes Care 32 (7): 1327–34. doi:10.2337/dc09-9033. PMID 19502545. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2699715
- "Diagnosis and classification of diabetes mellitus". Diabetes Care (American Diabetes Association) 33 Suppl 1 (Supplement_1): S62–69. January 2010. doi:10.2337/dc10-S062. PMID 20042775. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2797383
- "Diagnosis and classification of diabetes mellitus". Diabetes Care (American Diabetes Association) 35 Suppl 1 (Suppl 1): S64–71. January 2012. doi:10.2337/dc12-s064. PMID 22187472. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3632174
- Kumar, Vinay; Fausto, Nelson; Abbas, Abul K.; Cotran, Ramzi S.; Robbins, Stanley L. (2005). Robbins and Cotran Pathologic Basis of Disease (7th ed.). Philadelphia, Pa.: Saunders. pp. 1194–95. ISBN 0-7216-0187-1.
- Diabetes mellitus a guide to patient care.. Philadelphia: Lippincott Williams & Wilkins. 2007. p. 201. ISBN 978-1-58255-732-8. https://books.google.com/books?id=fiAclxvKblkC&pg=PA201.
- Vivian, Eva M.; Blackorbay, Brady (2013). "Chapter 13: Endocrine Disorders". in Lee, Mary. Basic Skills in Interpreting Laboratory Data (5th ed.). Bethesda, MD: American Society of Health-System Pharmacists. ISBN 978-1-58528-345-3. OCLC 859778842. https://books.google.com/books?id=5WW1AAAAQBAJ&pg=PT539.
- Valdez R (2009). "Detecting Undiagnosed Type 2 Diabetes: Family History as a Risk Factor and Screening Tool". Journal of Diabetes Science and Technology 3 (4): 722–26. doi:10.1177/193229680900300417. PMID 20144319. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2769984
- Selph, S; Dana, T; Blazina, I; Bougatsos, C; Patel, H; Chou, R (22 June 2015). "Screening for Type 2 Diabetes Mellitus: A Systematic Review for the U.S. Preventive Services Task Force". Annals of Internal Medicine 162 (11): 765–76. doi:10.7326/M14-2221. PMID 25867111. https://dx.doi.org/10.7326%2FM14-2221
- "Archived: Diabetes Mellitus (Type 2) in Adults: Screening". U.S. Preventive Services Task Force. June 2008. Archived from the original on 2014-02-07. https://web.archive.org/web/20140207214318/http://www.uspreventiveservicestaskforce.org/uspstf/uspsdiab.htm. Retrieved 2014-03-16.
- Siu, AL (27 October 2015). "Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement". Annals of Internal Medicine 163 (11): 861–68. doi:10.7326/M15-2345. PMID 26501513. https://dx.doi.org/10.7326%2FM15-2345
- "Draft Recommendation Statement Screening for Abnormal Glucose and Type 2 Diabetes Mellitus". http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/screening-for-abnormal-glucose-and-type-2-diabetes-mellitus. Retrieved 7 October 2014.
- "Standards of Medical Care in Diabetes – 2015: Summary of Revisions". Diabetes Care 54 (38): S4. 2015. doi:10.2337/dc15-S003. PMID 25537706. https://dx.doi.org/10.2337%2Fdc15-S003
- "Lifestyle interventions reduced the long-term risk of diabetes in adults with impaired glucose tolerance". Evidence-Based Medicine 13 (6): 173. December 2008. doi:10.1136/ebm.13.6.173. PMID 19043031. https://dx.doi.org/10.1136%2Febm.13.6.173
- Orozco LJ, Buchleitner AM, Gimenez-Perez G, Roqué I Figuls M, Richter B, Mauricio D (2008). Mauricio, Didac. ed. "Exercise or exercise and diet for preventing type 2 diabetes mellitus". Cochrane Database of Systematic Reviews (3): CD003054. doi:10.1002/14651858.CD003054.pub3. PMID 18646086. https://dx.doi.org/10.1002%2F14651858.CD003054.pub3
- Schellenberg, ES.; Dryden, DM.; Vandermeer, B.; Ha, C.; Korownyk, C. (October 2013). "Lifestyle Interventions for Patients With and at Risk for Type 2 Diabetes: A Systematic Review and Meta-analysis". Annals of Internal Medicine 159 (8): 543–51. doi:10.7326/0003-4819-159-8-201310150-00007. PMID 24126648. https://dx.doi.org/10.7326%2F0003-4819-159-8-201310150-00007
- O'Gorman, DJ; Krook, A (September 2011). "Exercise and the treatment of diabetes and obesity". Medical Clinics of North America 95 (5): 953–69. doi:10.1016/j.mcna.2011.06.007. PMID 21855702. https://dx.doi.org/10.1016%2Fj.mcna.2011.06.007
- Kyu, Hmwe H.; Bachman, Victoria F.; Alexander, Lily T.; Mumford, John Everett; Afshin, Ashkan; Estep, Kara; Veerman, J. Lennert; Delwiche, Kristen et al. (9 August 2016). "Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013". The BMJ 354: i3857. doi:10.1136/bmj.i3857. PMID 27510511. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4979358
- Nield L, Summerbell CD, Hooper L, Whittaker V, Moore H (2008). Nield, Lucie. ed. "Dietary advice for the prevention of type 2 diabetes mellitus in adults". Cochrane Database of Systematic Reviews (3): CD005102. doi:10.1002/14651858.CD005102.pub2. PMID 18646120. https://dx.doi.org/10.1002%2F14651858.CD005102.pub2
- Carter, P; Gray, LJ; Troughton, J; Khunti, K; Davies, MJ (2010-08-18). "Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis". The BMJ 341: c4229. doi:10.1136/bmj.c4229. PMID 20724400. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2924474
- "Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose" (PDF). Evidence Report/Technology Assessment (Summary) (128): 1–11. August 2005. PMID 16194123. PMC 4780988. Archived from the original on 2008-09-10. https://web.archive.org/web/20080910035155/http://www.ahrq.gov/downloads/pub/evidence/pdf/impglucose/impglucose.pdf.
- Haw, JS; Galaviz, KI; Straus, AN; Kowalski, AJ; Magee, MJ; Weber, MB; Wei, J; Narayan, KMV et al. (6 November 2017). "Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials". JAMA Internal Medicine 177 (12): 1808–17. doi:10.1001/jamainternmed.2017.6040. PMID 29114778. https://dx.doi.org/10.1001%2Fjamainternmed.2017.6040
- Seida, Jennifer C.; Mitri, Joanna; Colmers, Isabelle N.; Majumdar, Sumit R.; Davidson, Mayer B.; Edwards, Alun L.; Hanley, David A.; Pittas, Anastassios G. et al. (Oct 2014). "Effect of Vitamin D3 Supplementation on Improving Glucose Homeostasis and Preventing Diabetes: A Systematic Review and Meta-Analysis". The Journal of Clinical Endocrinology & Metabolism 99 (10): 3551–60. doi:10.1210/jc.2014-2136. PMID 25062463. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4483466
- "Type 2 diabetes: The management of type 2 diabetes". May 2009. Archived from the original on 2015-05-22. https://web.archive.org/web/20150522055955/http://www.nice.org.uk/guidance/cg87/chapter/1-recommendations#self-monitoring-of-plasma-glucose.
- Farmer, AJ; Perera, R; Ward, A; Heneghan, C; Oke, J; Barnett, AH; Davidson, MB; Guerci, B et al. (27 February 2012). "Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes". The BMJ 344: e486. doi:10.1136/bmj.e486. PMID 22371867. https://dx.doi.org/10.1136%2Fbmj.e486
- Emdin, CA; Rahimi, K; Neal, B; Callender, T; Perkovic, V; Patel, A (10 February 2015). "Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis". JAMA: the Journal of the American Medical Association 313 (6): 603–15. doi:10.1001/jama.2014.18574. PMID 25668264. https://dx.doi.org/10.1001%2Fjama.2014.18574
- McBrien, K; Rabi, DM; Campbell, N; Barnieh, L; Clement, F; Hemmelgarn, BR; Tonelli, M; Leiter, LA et al. (6 August 2012). "Intensive and Standard Blood Pressure Targets in Patients With Type 2 Diabetes Mellitus: Systematic Review and Meta-analysis". Archives of Internal Medicine 172 (17): 1–8. doi:10.1001/archinternmed.2012.3147. PMID 22868819. https://dx.doi.org/10.1001%2Farchinternmed.2012.3147
- Boussageon, R; Bejan-Angoulvant, T; Saadatian-Elahi, M; Lafont, S; Bergeonneau, C; Kassaï, B; Erpeldinger, S; Wright, JM et al. (2011-07-26). "Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials". The BMJ 343: d4169. doi:10.1136/bmj.d4169. PMID 21791495. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3144314
- Webster, MW (July 2011). "Clinical practice and implications of recent diabetes trials". Current Opinion in Cardiology 26 (4): 288–93. doi:10.1097/HCO.0b013e328347b139. PMID 21577100. https://dx.doi.org/10.1097%2FHCO.0b013e328347b139
- Inzucchi, SE; Bergenstal, RM; Buse, JB; Diamant, M; Ferrannini, E; Nauck, M; Peters, AL; Tsapas, A et al. (March 2015). "Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes.". Diabetologia 58 (3): 429–42. doi:10.1007/s00125-014-3460-0. PMID 25583541. https://dx.doi.org/10.1007%2Fs00125-014-3460-0
- Qaseem, Amir; Wilt, Timothy J.; Kansagara, Devan; Horwitch, Carrie; Barry, Michael J.; Forciea, Mary Ann (6 March 2018). "Hemoglobin A Targets for Glycemic Control With Pharmacologic Therapy for Nonpregnant Adults With Type 2 Diabetes Mellitus: A Guidance Statement Update From the American College of Physicians". Annals of Internal Medicine. doi:10.7326/M17-0939. https://dx.doi.org/10.7326%2FM17-0939
- Makam, AN; Nguyen, OK (10 January 2017). "An Evidence-Based Medicine Approach to Antihyperglycemic Therapy in Diabetes Mellitus to Overcome Overtreatment.". Circulation 135 (2): 180–95. doi:10.1161/CIRCULATIONAHA.116.022622. PMID 28069712. https://dx.doi.org/10.1161%2FCIRCULATIONAHA.116.022622
- Simpson, Terry C.; Weldon, Jo C.; Worthington, Helen V.; Needleman, Ian; Wild, Sarah H.; Moles, David R.; Stevenson, Brian; Furness, Susan et al. (2015-11-06). "Treatment of periodontal disease for glycaemic control in people with diabetes mellitus". Cochrane Database of Systematic Reviews (11): CD004714. doi:10.1002/14651858.CD004714.pub3. ISSN 1469-493X. PMID 26545069. https://dx.doi.org/10.1002%2F14651858.CD004714.pub3
- Smith, AD; Crippa, A; Woodcock, J; Brage, S (December 2016). "Physical activity and incident type 2 diabetes mellitus: a systematic review and dose-response meta-analysis of prospective cohort studies.". Diabetologia 59 (12): 2527–45. doi:10.1007/s00125-016-4079-0. PMID 27747395. https://dx.doi.org/10.1007%2Fs00125-016-4079-0
- Thomas, D. E.; Elliott, E. J.; Naughton, G. A. (2006-07-19). "Exercise for type 2 diabetes mellitus". The Cochrane Database of Systematic Reviews (3): CD002968. doi:10.1002/14651858.CD002968.pub2. ISSN 1469-493X. PMID 16855995. https://dx.doi.org/10.1002%2F14651858.CD002968.pub2
- "Exercise for the management of type 2 diabetes: a review of the evidence". Acta Diabetologica 47 (1): 15–22. March 2010. doi:10.1007/s00592-009-0126-3. PMID 19495557. https://link.springer.com/content/pdf/10.1007%2Fs00592-009-0126-3.pdf.
- "Nutritional strategies in type 2 diabetes mellitus". Mount Sinai Journal of Medicine 76 (3): 257–68. June 2009. doi:10.1002/msj.20118. PMID 19421969. https://dx.doi.org/10.1002%2Fmsj.20118
- Thomas D, Elliott EJ (2009). Thomas, Diana. ed. "Low glycaemic index, or low glycaemic load, diets for diabetes mellitus". Cochrane Database of Systematic Reviews (1): CD006296. doi:10.1002/14651858.CD006296.pub2. PMID 19160276. https://dx.doi.org/10.1002%2F14651858.CD006296.pub2
- Feinman, RD; Pogozelski, WK; Astrup, A; Bernstein, RK; Fine, EJ; Westman, EC; Accurso, A; Frassetto, L et al. (January 2015). "Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base.". Nutrition (Burbank, Los Angeles County, Calif.) 31 (1): 1–13. doi:10.1016/j.nut.2014.06.011. PMID 25287761. https://dx.doi.org/10.1016%2Fj.nut.2014.06.011
- Clifton, P (15 October 2017). "Assessing the evidence for weight loss strategies in people with and without type 2 diabetes.". World Journal of Diabetes 8 (10): 440–454. doi:10.4239/wjd.v8.i10.440. PMID 29085571. https://dx.doi.org/10.4239%2Fwjd.v8.i10.440
- "The health advantage of a vegan diet: exploring the gut microbiota connection". Nutrients 6 (11): 4822–38. 2014. doi:10.3390/nu6114822. PMID 25365383. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4245565
- Leach, Matthew J.; Kumar, Saravana (2012-09-12). "Cinnamon for diabetes mellitus". Cochrane Database of Systematic Reviews (9): CD007170. doi:10.1002/14651858.CD007170.pub2. ISSN 1469-493X. PMID 22972104. https://dx.doi.org/10.1002%2F14651858.CD007170.pub2
- Attridge, Madeleine; Creamer, John; Ramsden, Michael; Cannings-John, Rebecca; Hawthorne, Kamila (2014-09-04). "Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus". Cochrane Database of Systematic Reviews (9): CD006424. doi:10.1002/14651858.CD006424.pub3. ISSN 1469-493X. PMID 25188210. https://dx.doi.org/10.1002%2F14651858.CD006424.pub3
- Maruthur, NM; Tseng, E; Hutfless, S; Wilson, LM; Suarez-Cuervo, C; Berger, Z; Chu, Y; Iyoha, E et al. (19 April 2016). "Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis". Annals of Internal Medicine 164 (11): 740–51. doi:10.7326/M15-2650. PMID 27088241. https://dx.doi.org/10.7326%2FM15-2650
- Palmer, Suetonia C.; Mavridis, Dimitris; Nicolucci, Antonio; Johnson, David W.; Tonelli, Marcello; Craig, Jonathan C.; Maggo, Jasjot; Gray, Vanessa et al. (19 July 2016). "Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes". JAMA: the Journal of the American Medical Association 316 (3): 313–24. doi:10.1001/jama.2016.9400. PMID 27434443. https://dx.doi.org/10.1001%2Fjama.2016.9400
- Boussageon, R; Supper, I; Bejan-Angoulvant, T; Kellou, N; Cucherat, M; Boissel, JP; Kassai, B; Moreau, A et al. (2012). Groop, Leif. ed. "Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials". PLOS Medicine 9 (4): e1001204. doi:10.1371/journal.pmed.1001204. PMID 22509138. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3323508
- Zheng, Sean L.; Roddick, Alistair J.; Aghar-Jaffar, Rochan; Shun-Shin, Matthew J.; Francis, Darrel; Oliver, Nick; Meeran, Karim (17 April 2018). "Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes". JAMA 319 (15): 1580. doi:10.1001/jama.2018.3024. https://dx.doi.org/10.1001%2Fjama.2018.3024
- Richter, B; Bandeira-Echtler, E; Bergerhoff, K; Clar, C; Ebrahim, SH (18 July 2007). Richter, Bernd. ed. "Rosiglitazone for type 2 diabetes mellitus". Cochrane Database of Systematic Reviews (3): CD006063. doi:10.1002/14651858.CD006063.pub2. PMID 17636824. https://dx.doi.org/10.1002%2F14651858.CD006063.pub2
- Chen, X; Yang, L; Zhai, SD (December 2012). "Risk of cardiovascular disease and all-cause mortality among diabetic patients prescribed rosiglitazone or pioglitazone: a meta-analysis of retrospective cohort studies". Chinese Medical Journal 125 (23): 4301–06. PMID 23217404. http://www.ncbi.nlm.nih.gov/pubmed/23217404
- Lv, J; Perkovic, V; Foote, CV; Craig, ME; Craig, JC; Strippoli, GF (12 December 2012). Strippoli, Giovanni FM. ed. "Antihypertensive agents for preventing diabetic kidney disease". Cochrane Database of Systematic Reviews 12: CD004136. doi:10.1002/14651858.CD004136.pub3. PMID 23235603. https://dx.doi.org/10.1002%2F14651858.CD004136.pub3
- Cheng, J; Zhang, W; Zhang, X; Han, F; Li, X; He, X; Li, Q; Chen, J (May 2014). "Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis.". JAMA Internal Medicine 174 (5): 773–85. doi:10.1001/jamainternmed.2014.348. PMID 24687000. https://dx.doi.org/10.1001%2Fjamainternmed.2014.348
- Brunström, Mattias; Carlberg, Bo (24 February 2016). "Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses". The BMJ 352: i717. doi:10.1136/bmj.i717. PMID 26920333. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4770818
- Swinnen, SG.; Simon, AC.; Holleman, F.; Hoekstra, JB.; Devries, JH. (2011). Simon, Airin CR. ed. "Insulin detemir versus insulin glargine for type 2 diabetes mellitus". Cochrane Database of Systematic Reviews (7): CD006383. doi:10.1002/14651858.CD006383.pub2. PMID 21735405. https://dx.doi.org/10.1002%2F14651858.CD006383.pub2
- Waugh, N; Cummins, E; Royle, P; Clar, C; Marien, M; Richter, B; Philip, S (July 2010). "Newer agents for blood glucose control in type 2 diabetes: systematic review and economic evaluation". Health Technology Assessment (Winchester, England) 14 (36): 1–248. doi:10.3310/hta14360. PMID 20646668. https://dx.doi.org/10.3310%2Fhta14360
- Mirhosseini, Naghmeh; Vatanparast, Hassanali; Mazidi, Mohsen; Kimball, Samantha M (1 September 2017). "The Effect of Improved Serum 25-Hydroxyvitamin D Status on Glycemic Control in Diabetic Patients: A Meta-Analysis". The Journal of Clinical Endocrinology & Metabolism 102 (9): 3097–3110. doi:10.1210/jc.2017-01024. https://dx.doi.org/10.1210%2Fjc.2017-01024
- Picot, J; Jones, J; Colquitt, JL; Gospodarevskaya, E; Loveman, E; Baxter, L; Clegg, AJ (September 2009). "The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation". Health Technology Assessment (Winchester, England) 13 (41): iii–iv, 1–190, 215–357. doi:10.3310/hta13410. PMID 19726018. https://dx.doi.org/10.3310%2Fhta13410
- Frachetti, KJ; Goldfine, AB (April 2009). "Bariatric surgery for diabetes management". Current Opinion in Endocrinology, Diabetes and Obesity 16 (2): 119–24. doi:10.1097/MED.0b013e32832912e7. PMID 19276974. https://dx.doi.org/10.1097%2FMED.0b013e32832912e7
- Schulman, AP; del Genio, F; Sinha, N; Rubino, F (September–October 2009). ""Metabolic" surgery for treatment of type 2 diabetes mellitus". Endocrine Practice 15 (6): 624–31. doi:10.4158/EP09170.RAR. PMID 19625245. https://dx.doi.org/10.4158%2FEP09170.RAR
- Colucci, RA (January 2011). "Bariatric surgery in patients with type 2 diabetes: a viable option". Postgraduate Medicine 123 (1): 24–33. doi:10.3810/pgm.2011.01.2242. PMID 21293081. https://dx.doi.org/10.3810%2Fpgm.2011.01.2242
- Dixon, JB; le Roux, CW; Rubino, F; Zimmet, P (16 June 2012). "Bariatric surgery for type 2 diabetes". The Lancet 379 (9833): 2300–11. doi:10.1016/S0140-6736(12)60401-2. PMID 22683132. https://dx.doi.org/10.1016%2FS0140-6736%2812%2960401-2
- Rubino, F; Nathan, DM; Eckel, RH; Schauer, PR; Alberti, KG; Zimmet, PZ; Del Prato, S; Ji, L et al. (June 2016). "Metabolic Surgery in the Treatment Algorithm for Type 2 Diabetes: A Joint Statement by International Diabetes Organizations.". Diabetes Care 39 (6): 861–77. doi:10.2337/dc16-0236. PMID 27222544. https://dx.doi.org/10.2337%2Fdc16-0236
- GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015.". The Lancet 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMID 27733282. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=5055577
- "Ethnicity and type 2 diabetes: focus on Asian Indians". Journal of Diabetes and its Complications 15 (6): 320–27. 2001. doi:10.1016/S1056-8727(01)00161-1. PMID 11711326. https://dx.doi.org/10.1016%2FS1056-8727%2801%2900161-1
- Carulli, L; Rondinella, S; Lombardini, S; Canedi, I; Loria, P; Carulli, N (November 2005). "Review article: diabetes, genetics and ethnicity". Alimentary Pharmacology & Therapeutics 22 Suppl 2: 16–19. doi:10.1111/j.1365-2036.2005.02588.x. PMID 16225465. https://dx.doi.org/10.1111%2Fj.1365-2036.2005.02588.x
- Smyth, S; Heron, A (January 2006). "Diabetes and obesity: the twin epidemics". Nature Medicine 12 (1): 75–80. doi:10.1038/nm0106-75. PMID 16397575. https://dx.doi.org/10.1038%2Fnm0106-75
- "Global prevalence of diabetes: estimates for the year 2000 and projections for 2030". Diabetes Care 27 (5): 1047–53. May 2004. doi:10.2337/diacare.27.5.1047. PMID 15111519. https://dx.doi.org/10.2337%2Fdiacare.27.5.1047
- "Diabetes Fact sheet N°312". August 2011. Archived from the original on 26 August 2013. https://web.archive.org/web/20130826174444/http://www.who.int/mediacentre/factsheets/fs312/en/. Retrieved 2012-01-09.
- Leutholtz, Brian C.; Ripoll, Ignacio (2011). "Diabetes". Exercise and disease management (2nd ed.). Boca Raton: CRC Press. p. 25. ISBN 978-1-4398-2759-8. OCLC 725919496. https://books.google.com/books?id=eAn9-bm_pi8C&pg=PA25
- Zajac, Jacek; Shrestha, Anil; Patel, Parini; Poretsky, Leonid (2009). "The Main Events in the History of Diabetes Mellitus". in Poretsky, Leonid. Principles of diabetes mellitus (2nd ed.). New York: Springer. pp. 3–16. ISBN 978-0-387-09840-1. OCLC 663097550. https://books.google.com/books?id=i0qojvF1SpUC&pg=PA3
