Signal peptide, CUB, and EGF-like domain-containing proteins (SCUBE) are secretory cell surface glycoproteins that play key roles in the developmental process. SCUBE proteins participate in the progression of several diseases, including cancer, and are recognized for their oncogenic and tumor suppressor functions depending on the cellular context. SCUBE proteins promote cancer cell proliferation, angiogenesis, invasion, or metastasis, stemness or self-renewal, and drug resistance. The association of SCUBE with other proteins alters the expression of signaling pathways, including Hedgehog, Notch, TGF-β/Smad2/3, and β-catenin. Further, SCUBE proteins function as potential prognostic and diagnostic biomarkers for breast cancer, renal cell carcinoma, endometrial carcinoma, and nasopharyngeal carcinoma.
1. Emerging Role of SCUBE Family in Human Cancers
Aberrant expression of the SCUBE family plays a key role in cancer-cell proliferation, apoptosis, invasion, and migration in both in vitro and in vivo studies. SCUBE family proteins interact with other proteins and thus regulate their expression. SCUBE members positively modulate bone morphogenetic protein (BMP) signaling via inducing the specific interaction between BMP and BMP type I receptors affecting bone growth. Functionally, the SCUBE family maintains cellular signaling and regulates the expression of target genes via transcriptional or post-transcriptional mechanisms. The altered expression of SCUBE in cancer is linked to clinical outcome, highlighting the significant role that they may play as novel diagnostic and prognostic biomarkers, and as targets for cancer therapeutics.
1.1. Role of SCUBE as a Tumor Suppressor
SCUBE1 is associated with the proteins involved in angiogenesis, including platelet-derived growth factor D (PDGF-D), transforming growth factor-beta (TGF-β), and hedgehog (Hh). The mutations and mis-regulation of Hh signaling pathway components (PTCH1 and SMO) are associated with various cancer types [
9]. On the other hand, SCUBE2 expression is regulated and coordinated by two distinct mechanisms, namely, the BMP and β-catenin pathways [
18]. Antagonizing BMP and suppressing the β-catenin pathway exerts an inhibitory effect on breast-cancer cells. This phenomenon occurs on the C-terminal of the CUB domain that directly binds to the BMP and restricts its activity in an autocrine manner. In parallel, the N-terminal EGF-like repeats mediate cell-to-cell hemophilic adhesions in a calcium-dependent manner with further interaction with E-cadherin [
18]. Furthermore, SCUBE2 functions as a tumor suppressor in non small-cell lung cancer (NSCLC), glioma, and breast and colorectal cancer through the inhibition of the cell proliferation, migration, and invasion of tumor cells [
18,
22,
23,
24,
36]. Using bioinformatics analysis, a study demonstrated that the co-expression of SCUBE2 with N-acetyltransferase (NAT1) results in the alteration of several genes that play a critical role in triple-negative breast-cancer (TNBC) progression [
37,
38]. Another study identified the association of chromosome 19 miRNAs and SCUBE2 in the progression of TNBC subtype [
39]. Cheng et al. (2009) reported that altered SCUBE2 plays a significant role in breast-cancer cell proliferation and progression. This study revealed that the overexpression of the COOH terminal of SCUBE2 reduces MCF-7 breast-cancer cell growth. The overexpression of SCUBE2 protein functions as a BMP antagonist that suppresses cell proliferation in vitro and decreases tumor growth in an MCF-7 xenograft nude mouse model [
36]. Genome wide approaches such as methylated DNA immunoprecipitation (MEDIP) and whole-genome array analysis, in combination with high-density expression analysis, identified SCUBE3 as a frequently methylated and silenced gene in renal cell carcinoma (RCC), and thus a candidate tumor suppressor gene. A study reported that SCUBE3 tumor methylation was significantly associated with increased risk of cancer relapse and cancer-related death. The RNAi knockdown of SCUBE3 was associated with an advantage in anchorage-independent growth [
27].
1.2. SCUBE in Cell Proliferation and Cancer Progression
Accumulating evidence demonstrated that alteration in SCUBE expression promotes tumor growth in a cellular-context-dependent manner [
7,
9,
19,
22,
23,
24,
25,
26,
27]. Liang et al. (2015) reported the upregulation of SCUBE3 at both the transcript and the protein level in osteosarcoma cells (OS) and tissues compared with normal osteoblasts. Knockdown of SCUBE3 by siRNA significantly inhibited the proliferation of OS [
26]. A study reported the functional role of SCUBE3 in regulating the calcium ion pathway and their interactive association in human carcinogenesis through integrated network analysis. The group reported that lncRNA (CTB-113D17.1) competitively binds to hsa-miR-590-5p and facilitates SCUBE3 expression in OS [
40,
41]. Han et al. (2018) reported the overexpression of SCUBE3 in salivary adenoid cystic carcinoma (SACC) patients in comparison to a normal noncancerous gland. Dual-luciferase assay showed SCUBE3 to be a direct target of hsa-miR-885-5p [
25]. A recent study demonstrated an association between increased SCUBE3 expression and low E-cadherin levels in high histological-grade breast cancer, conforming a significant role of SCUBE3 in cancer progression and poor prognosis. The group also found an association of SCUBE3 expression with stage-specific breast tumors and subtypes. SCUBE3 expression was higher in TNBC in comparison to luminal, HER2+, and other breast-cancer subtypes [
42]. Another study reported that the forced expression of SCUBE1 reduces pro-tumorigenic activity in prostate-cancer-associated fibroblasts (CAFs) in a pre-clinical model [
43].
1.3. SCUBE in Regulating Angiogenesis
SCUBE proteins, primarily SCUBE2 upregulation, participate in the regulation of tumor angiogenesis. The process of the formation of new blood vessels is referred to as angiogenesis, which is critical for tumor growth and metastasis. In this context, VEGF interacts with VEGFR2 and activates endothelial cells that induce a cascade of expression of downstream molecules such as phospholipase C, PI3K/Akt, and γ-MAPK. These signaling cascades together promote endothelial cell proliferation, migration, and vessel formation [
7]. Lin et al. (2018) demonstrated the coreceptor performing activity of SCUBE2 with VEGFR2. Upon the induction of hypoxia, SCUBE2 promotes VEGF binding to its receptor to increase angiogenesis. EC-specific Scube2-knockout (EC-KO) and SP.B1 (anti-SCUBE2 monoclonal antibody) inhibited vascular tumor density and xenograft tumor growth, increased apoptosis, and reduced tumor-cell proliferation in a mouse model. The mechanism behind reducing tumor angiogenesis is the binding of SP.B1 to SCUBE2, which internalizes it to lysosomal degradation by preventing binding to VEGF and the activation of downstream signaling of VEGF. The binding of SP.B1 to SCUBE2 inhibited VEGFR2 phosphorylation and Akt/MAPK activation [
44]. This study was designed to develop anti-SCUBE2 therapy against tumor angiogenesis.
1.4. SCUBE in Invasion and Metastasis
Jiang et al. (2019) reported the relationship between SCUBE1 and miR-22 overexpression in a transcriptomic study. miR-22 acts as a tumor suppressor and has inhibitory effects on tumor growth, migration, and invasion. The group constructed miR-22-overexpressing cells found that SCUBE1 upregulation in the constructed cells is not the direct target of miR-22 and exhibited significantly increased apoptosis. Moreover, the study showed WRNIP1 to be a direct target of miR-22. The overexpression of miR-22 increases the radiosensitivity of NSCLC cells by targeting WRNIP1 [
45].
Yang et al. (2018) demonstrated the significant downregulation of SCUBE2 protein in NSCLC cell lines and human tissues. The group demonstrated that the forced expression of SCUBE2 markedly inhibited cell proliferation and induced apoptosis in NSCLC cells, whereas the upregulation of SCUBE2 altered NSCLC cell migration and invasion by inhibiting the sonic hedgehog signaling pathway. The forced expression of SCUBE2 altered the expression of the Smo, Shh, Gli1, and ptch1 proteins [
22]. Guo et al. (2017) reported low expression of SCUBE2 in glioma cell lines and tissues. The overexpression of SCUBE2 through transfection with pcDNA3.1-SCUBE2 into glioma U87 and A172 cells inhibited proliferation, migration, and invasion through the inhibition of the activity of the Shh signaling pathway. SCUBE2 overexpression substantially inhibited Gli1 expression in glioma cells. SCUBE2 plays a significant role in breast-cancer regulation [
24]. The expression of SCUBE2 and other breast-cancer-associated genes was clinically utilized to determine a predictive score in adjuvant guided treatment for breast-cancer patients [
15,
46,
47]. SCUBE2 expression regulates epithelial-to-mesenchymal (EMT) transition in breast-cancer cells. The ectopic expression of SCUBE2 reverses EMT transition and inhibits aggressiveness in breast-cancer cells (MDA-MB-231) by forming epithelial E-cadherin-containing adherens junctions. The cadherin-containing adherens junction is regulated by the β-catenin-SOX-mediated induction of FOXA1 (a positive regulator of E-cadherin). This mechanism decreases E-cadherin-mediated EMT transition in tumor cells. The direct expression of SCUBE2 is sufficient to inhibit TGF-β-mediated EMT induction in breast-cancer cells [
48]. Shen et al. (2020) identified the relationship among circular RNA circ_SETD2 (circ_SETD2), SCUBE2, and miR-155-5p in breast cancer. Both circ_SETD2 and SCUBE2 were downregulated, whereas miR-155-5p was upregulated in breast cancer. The overexpression of circ_SETD2 and SCUBE2 increases cell-cycle arrest, induces apoptosis, and inhibits cell proliferation, migration, and invasion in breast-cancer cells. Circ_SETD2 binds competitively to miR-155-5p and upregulate SCUBE2 expression, thereby inhibiting breast-cancer progression [
49]. SCUBE2 is downregulated in colorectal cancer. The low expression of SCUBE2 is associated with advanced clinical stage, higher histological-grade tumor, lymph nodes, and distant metastasis in colorectal-cancer (CRC) patients. The restoration of SCUBE2 reduced cell proliferation and colony formation, migration, and invasion in an in vitro model [
23]. The molecular mechanism behind SCUBE2 regulating CRC cell migration and invasion needs to be further evaluated. Wang et al. (2018) reported a lower expression of SCUBE2 at both the mRNA and protein levels in gastric cancer. The decreased expression of SCUBE2 is associated with lymph-node metastasis, high-grade tumors, advanced clinical stage, higher histological grade, distant-site metastasis, and vascular invasion [
50]. Parris et al. (2014) predicted the association of SCUBE2 with CBX2 and STK32B in the appearance of clinical–pathological characteristics, including increased tumor size, metastatic spread to cervical lymph nodes, and peritumoral inflammatory infiltration. Islam et al. (2016) identified that Shh signaling has the potential to increase tumorigenicity and stemness via EMT activation in bladder cancer through the switching and functional activation of CD133, Sox2, Nanog, and Oct4 [
51]. It would be interesting to know whether the SCUBE family plays a significant role in bladder-cancer progression. Furthermore, SCUBE2 functions as a tumor suppressor gene and is hypermethylated in breast tumors. Treatment with (-)-epigallocatechin-3-gallate (EGCG), a major polyphenolic constituent of green tea, significantly reversed SCUBE2 methylation by decreasing the expression of DNA methyltransferase (DNMT). EGCG-mediated increase in SCUBE2 expression inhibits vimentin expression, resulting in the suppression of breast-cancer cell migration and invasion [
52].
Upregulation of SCUBE3 was observed in NSCLC compared to adjacent normal tissues. SCUBE3 regulates the expression of several oncogenes in an autocrine or paracrine manner. SCUBE3 regulates the transcriptional and translational expression of the TGF-β/Smad2/3 signaling pathway responsible for the increased invasion of lung-cancer cells. MMP2 and MMP9 cleave SCUBE3 into two fragments
viz. N-terminal EGF-like repeats and the C-terminal CUB domain. C-terminal fragments having CUB domain either by themselves or together with TGF-β1 bind to the TGF-βII receptors and activate the TGF-β receptor/Smad2/3 signaling pathway. The activation of Smad2/3 through phosphorylation leads to the expression of downstream target genes. These include TGF-βI, slug and snail, MMP-2, MMP-9, PAI-1, and VEGF, which further promote epithelial-to-mesenchymal transition, invasion, metastasis, extracellular-matrix deposition, and angiogenesis in lung-cancer cells. SCUBE3 knockdown expression reduced the metastatic potential of NSCLC and tumor growth in an in vivo model [
35]. Microarray analysis revealed that SCUBE3 knockdown lowers the expression of genes controlling EMT and early angiogenesis in lung carcinoma [
53]. Zhao et al. (2013) investigated the clinical–pathological and prognostic significance of SCUBE3 in NSCLC tumors. Furthermore, the higher expression of SCUBE3 was positively correlated with advanced-stage tumors and lymph-node metastasis. The overexpression of SCUBE3 was also associated with the reduced expression of E-cadherin, and a higher expression of vimentin in NSCLC tumors [
54].
1.5. SCUBE in the Maintenance of Cancer Stemness and Drug Resistance
SCUBE2 functions as a novel breast tumor suppressor [
18] and possesses an oncogenic role in breast-cancer stem cells [
19]. The overexpression of SCUBE2 in breast-cancer stem cells increases TNBC aggressiveness via modulating the Stat3 and Notch signaling pathway. SCUBE2 upregulation increases the anchorage-independent cell growth, proliferation, migration, and invasion of tumor cells. SCUBE2 overexpression also increases cancer stem cell differentiation and self-renewal potential via an increase in the expression of ALDH1, Oct4, Sox2, and Nanog, and mammosphere formation, and reduces sensitivity to paclitaxel chemotherapy [
19]. Kallarakal et al. (2020) proposed SCUBE2, ELF5, and NFIB as a 3-signature gene panel biomarker to predict taxane-based neoadjuvant response in breast cancer [
55]. Recently, SCUBE2 was identified as a drug resistance gene that could be a promising tool for risk classification in ER-positive and HER2-negative breast-cancer patients [
56]. The expression of SCUBE family members in cancers and cancer stem cells, and their associated signaling pathways and targets is shown in
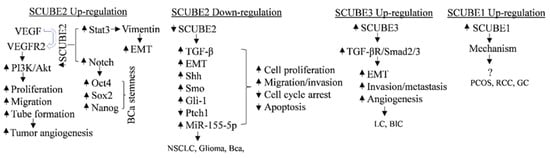
Figure 1.
Figure 1. Mechanism of SCUBE family in cancers and cancer stem cells in a contextual manner. VEGF, vascular endothelial growth factor; VEGFR2, vascular endothelial growth factor receptor 2; PI3K/Akt, phosphoinositide 3-kinase/protein kinase B; EMT, epithelial–mesenchymal transition; BCa, breast-cancer; Shh, sonic hedgehog; Ptch, patched homolog; Smo, smoothened; Gli, Gli family zinc finger; MMP-2/9, matrix metalloproteinase 2/9; Stat3, signal transducer and activator of transcription factor 3; TGF, transforming growth factor-β; NSCLC, nonsmall-cell lung cancer; LC, lung cancer; BlC, bladder cancer; PCOS, polycystic ovary syndrome; RCC, renal-cell cancer; GC, gastric cancer.
1.6. SCUBE and Immune Response
Erol et al. (2018) investigated the role of circulating SCUBE1 in lean, young, and glucose-tolerant women having polycystic ovary syndrome (PCOS), and healthy individuals. No significant association was noted between higher SCUBE 1 level and PCOS parameters. However, an increase in SCUBE1 was under the influence of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) [
57,
60]. Another study demonstrated that the lower expression of SCUBE1 in multiple myeloma patients could be responsible for an increased risk for arterial thrombosis, although the association remains un-established. The study concluded that lower expression of SCUBE1 may be possible due to defective platelets or increased TNF-α expression in multiple myeloma patients [
61].
1.7. SCUBE as a Biomarker
Cancer patients show an increased risk of developing thrombosis, and a continuous activation of the coagulation process. Some tumors have coagulation dysfunction that generates procoagulant substances [
62]. D-dimer is a well-known biomarker responsible for coagulation activation and fibrinolysis. D-dimer is independently associated with increased risk of thrombosis, which remains a major factor for breast-cancer-related deaths in women. SCUBE1 has been investigated in veins, arteries, and microvessels, and its higher expression was observed in the plasma of acute coronary and ischemic syndrome [
63]. Topcu et al. (2015) investigated the expression of SCUBE1 in breast-cancer patients and it compared with that of healthy individuals. SCUBE1 expression was significantly higher in the serum of HER2-positive breast-cancer patients than that in the HER2-negative phenotype. The group also identified a significantly higher expression of SCUBE1 and D-dimer in breast-cancer patients, suggesting SCUBE1 is responsible for hypercoagulability [
59]. Karagüzel et al. (2017) identified SCUBE1 as a promising biomarker in the diagnosis and prognosis of renal tumors. They identified a significant increase in the expression of SCUBE1 in renal = cell carcinoma compared with soluble urokinase plasminogen activator receptor and carbonic anhydrase IX in plasma samples [
9]. Mentese et al. (2012) demonstrated SCUBE1 to be a sensitive and specific biomarker for gastric cancer having higher SCUBE1 levels compared to those of the controls [
58]. Prognostic multigene expression profiles based on the levels of tumor gene expression estimated SCUBE2 along with 20 other signature genes as hallmarks of cancer-related variability in breast-cancer symptoms. This gene set was identified to affect pathways involved in cancer development and progression [
64]. Skrzypczak et al. (2013) reported significantly lower expression of SCUBE2 in high-grade endometrial cancer (G3) in comparison to the postmenopausal endometrium or G1 tumors. The study found a significant positive correlation between SCUBE2 transcription and PR and ERα, and PTEN expression might serve as a potential prognostic or predictive marker in endometrial cancer [
21]. Studies suggested SCUBE2 to be a prognostic and diagnostic biomarker in nasopharyngeal carcinoma and distant metastatic premenopausal patients [
65,
66]. SCUBE3 is also an independent poor prognostic factor in breast cancer [
42]. Joosten et al. (2017) reviewed SCUBE3 as a promising prognostic promoter methylation marker for renal-cell carcinoma [
67].
2. Role of SCUBE in Patient Survival
In a clinical study, SCUBE2 was suggested to have increased disease-free survival with poor overall survival in colorectal cancer [
23]. Another study reported SCUBE2 association with reduced survival in breast cancer [
68]. Kaplan–Meier data and univariate analysis demonstrated that colon-cancer patients with SCUBE2-positive tumors had better overall survival and disease-free survival in comparison to patients with SCUBE2-negative tumors. In gastric cancer, lower SCUBE2 expression exhibited significantly poor relapse-free survival and overall survival in patients [
50]. Ottley et al. (2020) demonstrated the association of luminal marker SCUBE2 and FOXA1 with disease-specific survival (DFS) in bladder cancer [
51]. Lower transcriptional and protein expression due to the hypermethylation of SCUBE2 has been reported in hepatocellular carcinoma patients. The lower methylation of SCUBE2-cg19000089 was significantly associated with better overall survival in HCC patients [
69]. NSCLC patients with high SCUBE3 expression had significantly less survival time in comparison to that of patients with low SCUBE3 expression [
54]. A study reported that the co-expression of Bcl2 and SCUBE2 is a notable factor for poor survival in estrogen-receptor-positive breast-cancer patients [
70]. Breast-cancer patients expressing SCUBE2 have better DFS but not OS in comparison to SCUBE2-negative patients. However, multivariate analysis showed that the expression of the SCUBE2 protein is an independent prognostic marker for disease-free survival [
71].
This entry is adapted from the peer-reviewed paper 10.3390/ijms231810577