Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Parkinson’s disease (PD) is a neurological disorder with complicated and disabling motor and non-motor symptoms. The complexity of PD pathology is amplified due to its dependency on patient diaries and the neurologist’s subjective assessment of clinical scales.
- Parkinson’s disease
- wearable devices
- digital health
1. Introduction
Parkinson’s disease (PD) is a complex neurodegenerative disorder that affects patients’ and their caregivers’ overall quality of life (QoL). Approximately 60,000 individuals in the United States are diagnosed with PD each year, while more than 10 million people are living with PD worldwide [1][2]. Many significant motor signs accompany PD, such as tremors [3][4], rigidity [5], bradykinesia [6][7][8][9], hypokinesia [10], postural instability [11][12], and gait difficulties [13][14][15]. While clinical diagnosis is usually based on these motor symptoms, non-motor symptoms are also common and sometimes more disabling than motor symptoms. Non-motor signs of PD include cognitive impairment [16][17], dementia [18], depression [19], and emotional changes [19][20].
The current practice for assessing the motor and non-motor symptoms of PD patients is a neurological examination, during which a neurologist watches the patient perform specific tasks [21]. Neurologists assign scores to the tasks performed by the patient as described by the Unified Parkinson’s Disease Rating Scale (UPDRS) [22] or its updated version, the Movement Disorder Society-sponsored revision of the UPDRS (MDS-UPDRS) [23]. Another rating scale, the Hoehn and Yahr scale (HY) [24] assigns patients an overall score between 0 and 5 based on their clinical stage. Since clinical assessments rely on descriptions of symptoms’ progression recorded in patient diaries, their credibility is limited by the subjectivity and recall bias of the patient [25][26]. Imaging equipment, such as magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET), can assist the neurologist in making an objective and more accurate diagnosis [27]. However, high equipment costs factor into the expenses, increasing the total cost [28]. Medication alone can cost USD 2500 a year, while corrective surgery, such as deep brain stimulation, costs up to USD 100,000 per person [1].
2. Application Areas
| Motor Signs and Symptoms |
Non-Motor Signs and Symptoms |
Mixed Signs and Symptoms |
| Gait | EEG abnormalities | |
| Limb movements | Cognitive activity | |
| EMG abnormalities | Depression | |
| FoG | Dementia | |
| Tremor | Heart rate | Speech topics |
| Activities of Daily Living (ADL) | Emotions | Swallowing |
| Bradykinesia and Dyskinesia | Fatigue | |
| Posture | Sleep topics | |
| Balance | Blinking | |
| Nocturnal Hypokinesia | Facial expression | |
| Handwriting | Breath | |
| Saccades | Cortical activity |
Table 1. The signs and symptoms assessed by technology in the current literature.
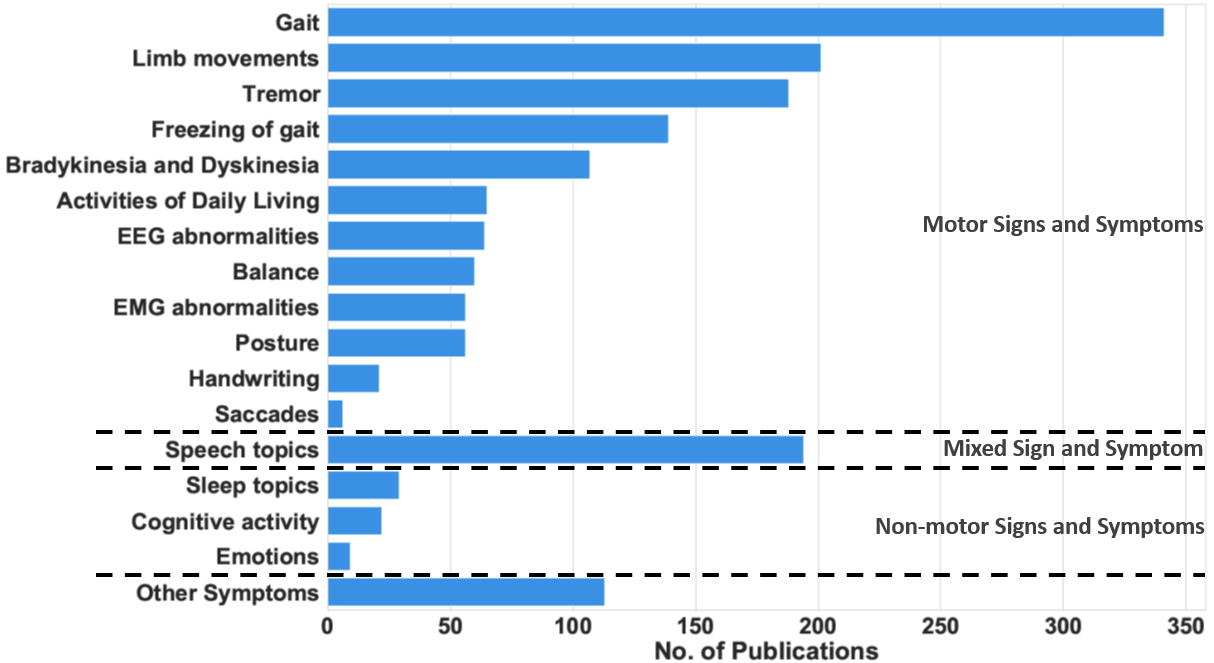 Figure 1. The number of research publications between 2008 and 2021 that measure each sign and symptom.
Figure 1. The number of research publications between 2008 and 2021 that measure each sign and symptom.Wearable and mobile technology can help medical practitioners assess the progression of PD patients by tracking their symptoms. These symptoms, referred to as cardinal PD features, are divided into motor symptoms and non-motor symptoms, as shown in Table 1. The motor symptoms are the movement impairments due to PD. They have traditionally been associated with tremor, rigidity, bradykinesia, and postural instability. Additionally, abnormalities in limb movements, such as hand rotation, finger tapping, and arm angle, have been regarded as motor symptoms. In contrast, non-motor symptoms include a large variety of cardinal features, such as sleep disturbance, cognitive activity, fatigue, dementia, and psychiatric impairments, as listed in the second column of Table 1. Finally, mixed symptoms, such as speech and swallowing difficulties, involve a combination of motor and non-motor activities. To show the prominence of these symptoms and justify their inclusion in our analysis, Figure 1 shows the number of articles that measure the different motor and non-motor symptoms. Gait abnormalities, including FoG, are the most common symptoms measured, followed by tremor. Movement problems, such as bradykinesia and dyskinesia, are also common symptoms in PD patients. Many research articles use modern measurement techniques to monitor these symptoms.
Our review and previous surveys of the PD literature [27][29] show that monitoring motor and non-motor symptoms can be helpful in four primary application areas in the PD research context: diagnosis, prognosis and monitoring, predicting response to treatment, and rehabilitation. The rest of this section reviews these areas.
PD diagnosis relies on neurologists’ clinical assessment of motor and non-motor symptoms [30]. To this end, neurologists observe patients while they perform specific tasks and assign them a score according to one of the standard scales: the previously mentioned UPDRS [22], its updated version, the Movement Disorder Society-sponsored revision of the UPDRS (MDS-UPDRS) [23], or the Hoehn and Yahr scale [24]. The clinical information derived from these rating scales is subjective. Hence, it leads to inter-rate variability and also intra-rate variability [27][29].
Moreover, as previously mentioned, the equipment used to supplement a clinical assessment tends to consist of expensive imaging tools, including MRI, SPECT, and PET. Because early and accurate diagnosis is essential, medical practitioners hope to augment current techniques with objective and cost-effective alternatives enabled by wearable and mobile technology. Therefore, recent research has examined the possibility of using wearable sensors and other portable technology to diagnose PD. These devices can provide objective PD-diagnosis measures that help standardize assessments [31][32][33]. Many researchers have also been able to use wearable sensors and other devices to distinguish PD patients from healthy controls in lab experiments [31][32][34].
In summary, wearable and mobile technology is used in the following sub-categories of PD diagnosis:
-
Early diagnosis of patients with PD;
-
Detecting PD symptoms in people with untreated PD;
-
Distinguishing PD patients from either healthy controls or patients with non-PD neurological disorders;
-
Distinguishing PD-related symptoms from similar symptoms not caused by PD (e.g., distinguishing PD tremors from essential tremors).
3. Prognosis and Monitoring the Severity of Symptoms
Predicting the patient’s future condition (prognosis) and assessing the severity of the disease, including current symptoms, are two activities that depend primarily on clinicians’ judgments and patients’ diary- or memory-based feedback. Clinicians’ judgments are subjective [27][29], while patients’ diary entries and memories are limited by compliance and recall bias [25][35][36]. Because clinical approaches that rely on these sources of information may not be completely reliable, the objective remote monitoring of PD symptoms holds immense promise for assessing the disease progression, evaluating symptom severity, and monitoring PD patients in unsupervised environments. To address these issues, recent work on PD prognosis and monitoring has focused on the following areas:
-
Home-based or remote monitoring of PD patients;
-
Evaluating the PD progression in a diagnosed patient;
-
Evaluating the severity of PD symptoms for a diagnosed patient.
4. Predicting Response to Treatment
To measure the efficacy of a treatment, clinicians rely on patients’ diary entries and unrecorded memories, both of which, as noted, can be subjective and unreliable. This problem has motivated researchers to measure the effects of treatments, including the effectiveness—and the side effects—with which medications suppress PD symptoms. To this end, researchers studying the ability of practitioners to accurately predict a patient’s response to treatment have been addressing the following issues:
-
Measuring the effects of such treatments as deep brain stimulation on the suppression of patients’ symptoms over time;
-
Measuring the intended effects of medications on patient symptoms;
-
Measuring the unintended effects (i.e., the side effects) of medications on patients (e.g., levodopa-induced dyskinesia).
5. Rehabilitation
Physiotherapy and other rehabilitation techniques are among the most common treatments for PD and, indeed, for movement disorders in general. As with medications, it is crucial to assess the efficacy of rehabilitation techniques. Additionally, it has been observed that cues and feedback are beneficial in assisting a PD patient. A rehabilitation system can provide auditory, visual, or haptic cues to facilitate a patient’s movement. Such a system can be explicitly used for gait training and assessing limb movements. Vibration-based actuators and audio feedback help patients suffering from rigidity, FoG, tremors, and other symptoms. These approaches can also help patients break out of a freeze and even suppress symptoms. Mobile technologies that target therapy and rehabilitation can be divided into the following sub-categories:
-
Audio, visual, or haptic cue for gait or movement training;
-
Sensory feedback to suppress a symptom such as FoG or tremor.
6. Technology in Parkinson’s Disease Research
Our review and other surveys of the PD literature [27][29][37][38] reveal that researchers use a number of device types and technologies in the application areas presented in the previous section. Using the current literature, we classify these devices into the following eight categories based on the device form factor and sensing modalities.
6.1. Wearable Devices
Many recent PD-oriented approaches focus on wearable devices for health monitoring since they help medical practitioners record patients’ activities and symptoms. Wearable devices are ideal monitoring technologies for two main reasons: they are not limited to a specific location and can be easily integrated into patients’ clothes [39]. The most commonly used wearable devices contain sensors, such as IMUs. The outputs of these sensors can facilitate analyses of body movement, gait, and symptoms, such as tremors. Our study covers the following wearable technologies:
-
IMUs with integrated accelerometers, gyroscopes, and magnetometer sensors;
-
Insole force- or pressure-based sensors that can measure the ground reaction force (GRF);
-
Wearable devices that use sensors such as EEG and EMG to measure neural responses and muscle activities;
-
Clothing-integrated sensors, such as strain or accelerometer sensors, that measure hand tremors;
-
Other wearable devices, including smart glasses and smart hats, that can record patients’ emotions and other specific parameters.
6.2. Biopotential Devices
These devices can measure the electrical signals (biopotentials) generated by our body’s physiological processes. This category includes electroencephalogram, electrocardiogram, magnetoencephalogram, and electrooculogram devices. They can be either standalone devices, such as a 16-lead EEG system, or wearable devices with a single-lead EMG sensor. When a biopotential device is integrated into a wearable device, the resulting device falls under both the wearable and biopotential-device categories.
6.3. Cueing Devices
Devices that give feedback or cues to patients to rectify their walk or assist with other movements fall under this category. Headphones or speakers can deliver auditory cues, and visual cues can appear on a screen, smart glass, or even in a virtual-reality environment. Vibration sensors and electrical stimulators also can give sensory feedback to patients.
6.4. Optical Motion Tracker
These devices track user motion with either radio frequency or optical signals. For example, motion-capturing systems such as Microsoft Kinect [40] and Vicon 3D [41] use structured light for analysis. Similarly, radar-based systems use radio-frequency signals to monitor the motion of subjects [42][43]. A system of multiple IMUs, even camera-based 3D setups, can also capture a patient’s movement.
6.5. Audio Recording
Many PD patients experience issues with swallowing or speech, as shown in Table 1. Audio recording devices can analyze and monitor symptoms related to swallowing and speech. For instance, microphones and smartphones record speech tasks or patients’ voices for the subsequent diagnostic analysis [34][44].
6.6. Video Recording
Video-recording devices are helpful for PD monitoring because gait and motor issues are among the most common PD symptoms. For instance, video cameras record patients’ movements at home or during laboratory experiments, and practitioners can use the recordings to spot symptoms or to corroborate or disprove predictions based on other devices [6][20][45].
6.7. Force/Pressure
Force or pressure plates measure the force exerted by the patients’ feet while walking. Therefore, they help measure the gait quality of the patient. Force or pressure sensors are also integrated into gait mats to measure the gait quality of a patient.
6.8. Smartphone
Researchers have used accelerometers, gyroscopes, magnetometers, GPS, and other sensors on smartphones for various analytical objectives. Smartphone applications can also record such matters as patients’ emotional displays and drug dosages. Additionally, smartphone screens can record handwriting, microphones can record speech, and cameras can record movement.
6.9. Other
Other devices have also helped practitioners assess PD patients. We classify these devices that do not fall into the above categories as “other.” For example, digitized tablets can serve as smart screens for handwriting assessment. PD patients can write on these screens during spiralography exams. Likewise, smart pens can record hand movements during writing. Solutions based on virtual or augmented reality have also started becoming popular in PD applications.
This entry is adapted from the peer-reviewed paper 10.3390/s22155491
References
- The Parkinson’s Foundation. Parkinson’s Foundation: Better Lives Together. Available online: https://bit.ly/2Uc6ikj (accessed on 25 June 2022).
- Marras, C.; Beck, J.; Bower, J.; Roberts, E.; Ritz, B.; Ross, G.; Abbott, R.; Savica, R.; Van Den Eeden, S.; Willis, A.; et al. Prevalence of Parkinson’s disease across North America. NPJ Park. Dis. 2018, 4, 21.
- Harish, K.; Rao, M.V.; Borgohain, R.; Sairam, A.; Abhilash, P. Tremor quantification and its measurements on parkinsonian patients. In Proceedings of the 2009 International Conference on Biomedical and Pharmaceutical Engineering, Singapore, 2–4 December 2009; pp. 1–3.
- Contreras, R.; Huerta, M.; Sagbay, G.; LLumiguano, C.; Bravo, M.; Bermeo, A.; Clotet, R.; Soto, A. Tremors quantification in parkinson patients using smartwatches. In Proceedings of the 2016 IEEE Ecuador Technical Chapters Meeting (ETCM), Guayaquil, Ecuador, 12–14 October 2016; pp. 1–6.
- Jenkins, M.; Almeida, Q.; Spaulding, S.; Van Oostveen, R.; Holmes, J.; Johnson, A.M.; Perry, S. Plantar cutaneous sensory stimulation improves single-limb support time, and EMG activation patterns among individuals with Parkinson’s disease. Park. Relat. Disord. 2009, 15, 697–702.
- Zwartjes, D.G.; Heida, T.; Van Vugt, J.P.; Geelen, J.A.; Veltink, P.H. Ambulatory monitoring of activities and motor symptoms in Parkinson’s disease. IEEE Trans. Biomed. Eng. 2010, 57, 2778–2786.
- George, J.S.; Strunk, J.; Mak-McCully, R.; Houser, M.; Poizner, H.; Aron, A.R. Dopaminergic therapy in Parkinson’s disease decreases cortical beta band coherence in the resting state and increases cortical beta band power during executive control. NeuroImage Clin. 2013, 3, 261–270.
- Teskey, W.J.; Elhabiby, M.; El-Sheimy, N. Inertial sensing to determine movement disorder motion present before and after treatment. Sensors 2012, 12, 3512–3527.
- Bayés, À.; Samá, A.; Prats, A.; Pérez-López, C.; Crespo-Maraver, M.; Moreno, J.M.; Alcaine, S.; Rodriguez-Molinero, A.; Mestre, B.; Quispe, P.; et al. A “HOLTER” for Parkinson’s disease: Validation of the ability to detect on-off states using the REMPARK system. Gait Posture 2018, 59, 1–6.
- Bhidayasiri, R.; Sringean, J.; Anan, C.; Boonpang, K.; Thanawattano, C.; Chaudhuri, K.R. Quantitative demonstration of the efficacy of night-time apomorphine infusion to treat nocturnal hypokinesia in Parkinson’s disease using wearable sensors. Park. Relat. Disord. 2016, 33, S36–S41.
- Mancini, M.; Salarian, A.; Carlson-Kuhta, P.; Zampieri, C.; King, L.; Chiari, L.; Horak, F.B. ISway: A sensitive, valid and reliable measure of postural control. J. Neuroeng. Rehabil. 2012, 9, 59.
- Phan, D.; Horne, M.; Pathirana, P.; Farzanehfar, P. Measurement of Axial Rigidity and Postural Instability Using Wearable Sensors. Sensors 2018, 18, 495.
- Baram, Y.; Lenger, R. Gait improvement in patients with cerebral palsy by visual and auditory feedback. Neuromodulation Technol. Neural Interface 2012, 15, 48–52.
- Mileti, I.; Germanotta, M.; Alcaro, S.; Pacilli, A.; Imbimbo, I.; Petracca, M.; Erra, C.; Di Sipio, E.; Aprile, I.; Rossi, S.; et al. Gait partitioning methods in Parkinson’s disease patients with motor fluctuations: A comparative analysis. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rochester, MN, USA, 7–10 May 2017; pp. 402–407.
- De Lima, A.L.S.; Evers, L.J.; Hahn, T.; Bataille, L.; Hamilton, J.L.; Little, M.A.; Okuma, Y.; Bloem, B.R.; Faber, M.J. Freezing of gait and fall detection in Parkinson’s disease using wearable sensors: A systematic review. J. Neurol. 2017, 264, 1642–1654.
- Güntekin, B.; Hanoğlu, L.; Güner, D.; Yılmaz, N.H.; Çadırcı, F.; Mantar, N.; Aktürk, T.; Emek-Savaş, D.D.; Özer, F.F.; Yener, G.; et al. Cognitive impairment in parkinson’s disease is reflected with gradual decrease of EEG delta responses during auditory discrimination. Front. Psychol. 2018, 9, 170.
- Ghosh, L.; Parui, S.; Rakshit, P.; Konar, A. EEG analysis for working memory modeling in face recognition task. In Proceedings of the 2017 Third International Conference on Research in Computational Intelligence and Communication Networks (ICRCICN), Kolkata, India, 3–5 November 2017; pp. 33–38.
- Garn, H.; Coronel, C.; Waser, M.; Caravias, G.; Ransmayr, G. Differential diagnosis between patients with probable Alzheimer’s disease, Parkinson’s disease dementia, or dementia with Lewy bodies and frontotemporal dementia, behavioral variant, using quantitative electroencephalographic features. J. Neural Transm. 2017, 124, 569–581.
- Dietz, J.; Bradley, M.; Jones, J.; Okun, M.; Perlstein, W.; Bowers, D. The late positive potential, emotion and apathy in Parkinson’s disease. Neuropsychologia 2013, 51, 960–966.
- Ricciardi, L.; Bologna, M.; Morgante, F.; Ricciardi, D.; Morabito, B.; Volpe, D.; Martino, D.; Tessitore, A.; Pomponi, M.; Bentivoglio, A.R.; et al. Reduced facial expressiveness in Parkinson’s disease: A pure motor disorder? J. Neurol. Sci. 2015, 358, 125–130.
- Deb, R. How Does Technology Development Influence the Assessment of Parkinson’s Disease? A Systematic Review. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2019.
- Fahn, S. UPDRS Development Comittee. Unified Parkinson’s disease rating scale. Recent Dev. Park. Dis. 1987, 2.
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170.
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1998, 50, 318.
- Ozanne, A.; Johansson, D.; Hällgren Graneheim, U.; Malmgren, K.; Bergquist, F.; Alt Murphy, M. Wearables in epilepsy and Parkinson’s disease—A focus group study. Acta Neurol. Scand. 2018, 137, 188–194.
- Bhat, G.; Deb, R.; Ogras, U.Y. OpenHealth: Open-source platform for wearable health monitoring. IEEE Des. Test 2019, 36, 27–34.
- Yang, K.; Xiong, W.X.; Liu, F.T.; Sun, Y.M.; Luo, S.; Ding, Z.T.; Wu, J.J.; Wang, J. Objective and quantitative assessment of motor function in Parkinson’s disease—from the perspective of practical applications. Ann. Transl. Med. 2016, 4, 90.
- Boland, D.F.; Stacy, M. The economic and quality of life burden associated with Parkinson’s disease: A focus on symptoms. Am. J. Manag. Care 2012, 18, S168–S175.
- Rovini, E.; Maremmani, C.; Cavallo, F. How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front. Neurosci. 2017, 11, 555.
- Kassubek, J. Diagnostic procedures during the course of Parkinson’s Disease. Basal Ganglia 2014, 4, 15–18.
- Yoneyama, M.; Kurihara, Y.; Watanabe, K.; Mitoma, H. Accelerometry-Based gait analysis and its application to parkinson’s disease assessment—Part 2: A new measure for quantifying walking behavior. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 999–1005.
- Meigal, A.I.; Rissanen, S.; Tarvainen, M.; Karjalainen, P.; Iudina-Vassel, I.; Airaksinen, O.; Kankaanpää, M. Novel parameters of surface EMG in patients with Parkinson’s disease and healthy young and old controls. J. Electromyogr. Kinesiol. 2009, 19, e206–e213.
- Ferri, R.; Fulda, S.; Cosentino, F.I.; Pizza, F.; Plazzi, G. A preliminary quantitative analysis of REM sleep chin EMG in Parkinson’s disease with or without REM sleep behavior disorder. Sleep Med. 2012, 13, 707–713.
- Camnos-Roca, Y.; Calle-Alonso, F.; Perez, C.J.; Naranjo, L. Computational Diagnosis of Parkinson’s Disease from Speech Based on Regularization Methods. In Proceedings of the 2018 26th European Signal Processing Conference (EUSIPCO), Rome, Italy, 3–7 September 2018; pp. 1127–1131.
- Stone, A.A.; Shiffman, S.; Schwartz, J.E.; Broderick, J.E.; Hufford, M.R. Patient compliance with paper and electronic diaries. Control. Clin. Trials 2003, 24, 182–199.
- Papapetropoulos, S. Patient diaries as a clinical endpoint in Parkinson’s disease clinical trials. CNS Neurosci. Ther. 2012, 18, 380–387.
- Forte, R.; Tocci, N.; De Vito, G. The Impact of Exercise Intervention with Rhythmic Auditory Stimulation to Improve Gait and Mobility in Parkinson Disease: An Umbrella Review. Brain Sci. 2021, 11, 685.
- Ponciano, V.; Pires, I.M.; Ribeiro, F.R.; Marques, G.; Villasana, M.V.; Garcia, N.M.; Zdravevski, E.; Spinsante, S. Identification of diseases based on the use of inertial sensors: A systematic review. Electronics 2020, 9, 778.
- Bhat, G.; Tran, N.; Shill, H.; Ogras, U.Y. w-HAR: An Activity Recognition Dataset and Framework Using Low-Power Wearable Devices. Sensors 2020, 20, 5356.
- Lachat, E.; Macher, H.; Landes, T.; Grussenmeyer, P. Assessment and calibration of a RGB-D camera (Kinect v2 Sensor) towards a potential use for close-range 3D modeling. Remote Sens. 2015, 7, 13070–13097.
- Vicon. Vicon Motion Capture System. Available online: https://www.vicon.com (accessed on 25 June 2022).
- An, S.; Ogras, U.Y. MARS: mmWave-based Assistive Rehabilitation System for Smart Healthcare. ACM Trans. Embed. Comput. Syst. TECS 2021, 20, 1–22.
- An, S.; Ogras, U.Y. Fast and Scalable Human Pose Estimation using mmWave Point Cloud. arXiv 2022, arXiv:2205.00097.
- Tsanas, A.; Little, M.A.; McSharry, P.E.; Spielman, J.; Ramig, L.O. Novel speech signal processing algorithms for high-accuracy classification of Parkinson’s disease. IEEE Trans. Biomed. Eng. 2012, 59, 1264–1271.
- Patel, S.; Lorincz, K.; Hughes, R.; Huggins, N.; Growdon, J.; Standaert, D.; Akay, M.; Dy, J.; Welsh, M.; Bonato, P. Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 864–873.
This entry is offline, you can click here to edit this entry!
