Gout is a metabolic disorder, and one of the most common inflammatory arthritic conditions, caused by elevated serum urate (SU). Gout prevalence is globally rising, partly due to global dietary changes and the growing older adult population. Gout was known to affect people of high socioeconomic status. Currently, gout disproportionately affects specific population subgroups that share distinct racial and ethnic backgrounds. While genetics may predict SU levels, nongenetic factors, including diet, cultural traditions, and social determinants of health (SDOH), need to be evaluated to optimize patient treatment outcomes. A cultural assessment may inform the development of culturally tailored dietary recommendations for patients with gout. Causal and association studies investigating the interaction between diet, genetics, and gout, should be cautiously interpreted due to the lack of reproducibility in different racial groups. Optimal gout management could benefit from a multidisciplinary approach, involving pharmacists and nurses.
- acculturation
- gout management
- hyperuricemia
- culture
- diet
- genetics
1. Introduction
Gout is a metabolic disorder, and one of the most common inflammatory arthritic conditions worldwide, caused by persistent hyperuricemia. Developing gout is multifactorial, ushering in different methodological approaches to ascertain the risk factors associated with developing hyperuricemia and gout. Despite substantial advancement in understanding the biological basis of gout, it remains one of the most poorly managed chronic conditions in healthcare. Uncontrolled gout is associated with a poor quality of life, joint damage, an increase in missed days of work, and a higher utilization of the healthcare system resources [1,2,3].
Gout is a chronic inflammatory condition caused by persistent hyperuricemia, leading to the formation and deposition of monosodium urate crystals into and around the distal joints. The development of hyperuricemia and gout is heterogenous, and, therefore, different research approaches are needed to identify and quantify the distinct risk factors in the pathogenesis of both conditions. For example, the Mendelian Randomization (MR) approach provides a pathway to ascertain causality, exploiting the natural randomization of allele causal disease. However, this approach is not without limitations, possibly due to the pleiotropic effect of the selected instrumental variables [20]. As gout continues to disproportionately affect non-EUR populations, there is a growing need to increase the representation of minorities in genetic research and cross-validation of genetic findings in multiple populations. To that end, we recognized that developing hyperuricemia and gout is a multifactorial process founded in genetics and modulated by epigenetic factors, including medications, lifestyle factors, diet, and the potential interactions between all of them. While genetic polymorphisms in ABCG2 and SLC2A9 remain two of the most significant signals in developing hyperuricemia and gout across different populations, evaluating nongenetic factors across selected populations through a cultural lens is an adjunct approach to further stratify hyperuricemia and gout risk and optimize gout management. This encompassing approach could be a valuable tool for gout patients with strong cultural identities and distinct racial or ethnic backgrounds. A summary of the major genes associated with regulating uric acid in humans is listed in Table 1.
| Gene | Protein | Possible Functions |
|---|---|---|
| ABCG2 | ATP binding cassette subfamily G member 2: ABCG2 | Regulating renal and gut excretion of urate. Gene polymorphisms are strongly linked to urate underexcretion and the risk of early-onset gout in men. Genetic polymorphisms may also influence the therapeutic response to allopurinol and other statin medications. |
| GCKR | Glucokinase regulator | Regulatory protein that inhibits glucokinase in the liver and pancreatic islet cells by forming an inactive complex with the enzyme. Gene polymorphisms are associated with fasting glucose, maturity-onset type-2 diabetes, hyperuricemia, and gout. |
| LRRC16A | Capping protein regulator and myosin 1 linker 1: CARMIL1 | Cytoskeleton-associated protein. Gene polymorphisms are associated with urate concentrations and gout subtypes. |
| PDZK1 | PDZK domain-containing scaffolding protein | Mediates the localization of cell surface proteins and plays a critical role in cholesterol metabolism. Gene polymorphisms are linked to dyslipidemia, hyperuricemia, and gout. |
| SLC2A9 | Solute carrier family 2 member 9: GLUT9 | Regulating renal uric acid reabsorption. Gene polymorphisms are linked to the risk of gout in women. |
| SLC16A9 | Solute carrier family 16 member 9: MCT9 | Regulating monocarboxylic acid transporter. Gene polymorphisms are linked to uric acid concentrations. |
| SLC17A1 | Solute carrier family 17 member 1: NPT1 | Sodium phosphate cotransporter. Gene polymorphisms are linked with hyperuricemia and gout. |
| SLC22A11 | Solute carrier family 22 member 11: OAT4 | Urate reabsorption transporter. A target for some uricosuric drugs. Gene polymorphisms are associated with hyperuricemia. |
| SLC22A12 | Solute carrier family 22 member 12: URAT1 | Uric acid reabsorption transporter. A major target for uricosuric drugs. Gene polymorphisms are associated with hyperuricemia and gout. Loss of function in the gene can also lead to hypouricemia. |
2. Heritability of Urate Levels and Urate-Modifying Factors
| Diet/Food/Lifestyle Factor | Serum Urate Level | Incident Gout | Gout Flare Risk | ACR 2020 Recommendations [30] | References |
|---|---|---|---|---|---|
| DASH diet |  |
 |
 |
No recommendation | [31,32,33] |
| Mediterranean diet |  |
 |
 |
No recommendation | [34] |
| Ketogenic diet |  |
No data | No data | No recommendation | [35] |
| Low-fat dairy products |
 |
 |
 |
No recommendation | [36,37] |
| Cherries |  |
 |
 |
No recommendation | [38,39] |
| Coffee |   |
 |
 |
No recommendation | [40,41,42,43] |
| Tea |   |
No data | No data | No recommendation | [42,43,44] |
| High-fructose corn syrup (HFCS) |  |
 |
 |
Conditionally recommends limiting the intake of HFCS | [15,19] |
| Weight loss |  |
 |
 |
Conditionally recommends a weight loss program | [45,46] |
| Physical exercise |  |
No data | No data | No recommendation | [26,45] |
| Smoking |   |
 |
No data | No recommendation | [47,48,49] |
| Alcohol |  |
 |
 |
Conditionally recommends limiting alcohol intake | [50,51,52] |
| Vitamin B complex (B6-B12-Folic acid) |  |
No data | No data | No recommendation | [53] |
| Vitamin C |   |
No data | 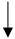 |
Conditionally recommends against use | [27,54,55] |
| Fish Oil/Omega-3-fatty acids |  |
No data | 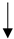 |
No recommendation | [28,56,57] |
3. Gout Risk and Acculturation
Dietary habits are mirrors of cultural customs and traditions. Certain population subgroups tend to follow a specific diet for personal, social, and religious norms [11,58]. Nonetheless, immigration or acculturation could significantly impact the lifestyle and dietary habits of the same population groups [58,59,60]. These changes could have significant effects on the individuals’ overall health, ranging from energy expenditure-related activities to their gut microbiome. Collectively, these changes could have a consequential impact on developing cardiometabolic risk factors, including hyperuricemia and gout. Similarly, Filipinos living in the US were reported to have higher gout rates and elevated means SU levels than those residing in the Philippines, suggesting significant gene–environment interactions [62,63]. Conversely, developing hyperuricemia or gout among US immigrant groups could be owing to a more permissive environment, increasing the penetrance of risk alleles by equivalent effects [58,64]. For example, the joint effect of alcohol consumption and carrying the risk allele of ABCG2 rs2231142 G > T was associated with a greater risk for developing hyperuricemia than the risk allele alone, especially among women [65]. Therefore, ascertaining the dietary and social lifestyle habits among distinct racial and ethnic groups could shed additional light on the hypothesis of gene–diet/gene–social habits interactions and population-specific risk for developing hyperuricemia or gout [50,66]. Additionally, this ascertainment may provide more evidence on the role of preserving a cultural identity in disease prevention and the generational effect on disease onset among immigrant groups.4. Gout Risk and Health Beliefs
5. Gout and Social Determinants of Health
6. Multidisciplinary Approach to Gout Management
7. Pharmacogenomics and Gout Management
This entry is adapted from the peer-reviewed paper 10.3390/nu14173590
