Spatholobus suberectus Dunn (SSD, Leguminosae) is a perennial woody vine, indigenous to tropical and subtropical forests in China and other Southeast Asian countries. The vine stem of SSD is called “Jixueteng” (literally means ‘chicken blood vines’) in Chinese, due to the blood-like outflow from its vine stem when it is injured. SSD has been extensively employed in Traditional Chinese Medicine to treat several ailments. SSD and its active compounds are effective therapeutic agents for treating a variety of diseases with negligible side effects. SSD has been frequently attributed to having antioxidant, anti-diabetic, anti-inflammatory, hematopoietic, neuroprotective, antimicrobial, and anticancer properties.
- Spatholobus suberectus Dunn
- phytochemistry
- pharmacological activity
- cancer prevention
1. Introduction
The vine stem of Spatholobus suberectus Dunn (SSD, Leguminosae) has been widely employed in Chinese Medicine to treat hematopoiesis, anemia, rheumatism, and menoxenia in Chinese society for nearly years [1]. (Figure 1). It is most commonly used as a food additive in southern Asian regions for wine, tea, and soup. In clinical practice, herbal medicine is highly effective in terms of its bioavailability and dose effectiveness, as it contains active ingredients with reliable bioavailability. The crude extract from SSD is thus used in a number of patented Chinese medicines, and the demand for the crude resource is increasing rapidly today. However, unlike other legume families, the seedling growth proportion of SSD is relatively weak; the fruit drops off too early, resulting in poor reproductive ability [2]. SSD usually takes 7 years to develop and yield effective drugs, which can then be used as medicines. Over the past decades, SSD has been recognized as an endangered plant species and has been documented on the Red List of Biodiversity in China, as a consequence of its low growth rate and man-made activities, including deforestation and overexploitation [3]. Furthermore, the market demand for SSD is massive, and the wild resources can no longer meet these mounting requirements. Thus, the Ministry of Ecology and Environment encourages the artificial planting of SSD in highland areas of Xishuangbanna, southwest Yunnan, China. This area is characterized by a distinct climate, sporadic rainy events, and dry seasons, which can be used in order to cultivate surplus numbers of SSD.

2. Phytochemistry
SSD comprises distinguished polyphenol compounds, including flavonoids, carotenoids, chalcone, coumestans, lignans, and phenolic acids [4]. The phenolic contents of SSD are relatively higher than that of many other medicinal plants [5][6][7]. Several analytical methods have been used to isolate, purify, and characterize the chemical compounds from SSD [8][9][10][11]. Several bioactive chemicals in SSD can be identified by HPLC chromatographic fingerprinting. The polyamide column chromatography method is also used to separate polyphenols in SSD. This is followed by the HPLC method to determine the flavonoid components, which enables quality control assurance in identifying the compounds in SSD [12]. Ultraviolet spectrophotometry applications in vanillin–acid assays and the aluminum nitrate colorimetric method are also employed to determine the content of condensed tannins and total flavonoids in SSD [13][14][15][16].
About 57 chemical ingredients in the SSD stem were isolated by the effective method of combining Ultra-Fast Liquid Chromatography and Tandem Mass Spectrometry. The content of chemicals detected in the descending order was isoflavones, flavanols, phenolic acid esters, terpenoids, lignans, and coumarins. Among a pool of chemicals, (-)-gallocatechin, (-)-catechin, (-)-epicatechin, biochanin A, (-)-epiafzelechin, 4,7,2′-trihydroxy-4′-methoxyisoflavanol, β-sitosterol, dihydrocajanin, and maackiain exhibited the highest concentrations in crude extracts [17]. Different analytical techniques can be used to determine the content of bioactive compounds that distinguish the quality and quantity of SSD. A total of 16 compounds were found in SSD as identified by HPLC-MS/MS [18]. Four active flavonoids, viz., protocatechuic acid, catechin, gallocatechin, and formononetin, were determined in the plasma of rats by a UPLC-MS/MSn method, and these active principles were successfully employed in a pharmacokinetics investigation employed after the oral administration of SSD [19]. Diversified compounds from SSD have been identified and classified based on the class, and subclass.3. Pharmacological Activity of SSD
3.1. Antioxidant Activity
3.2. Antidiabetic Activity
3.3. Anti-Inflammatory Activity
It has been discovered that a number of inflammatory reactions are triggered by a mucosal injury, microbial infection, and oxidative stress, leading to the identification of pathophysiological mechanisms that are to detect noxious agents infecting the host, such as lipopolysaccharide (LPS) [30][34]. LPS is an antigen of the bacterial cell, which induces macrophage activation, causing excessive NO synthesis, inflammatory mediators, prostaglandins, TNF-α, and several pro-inflammatory cytokines [35][36]. A possible method for treating inflammatory diseases is to attenuate the production of these inflammatory mediators.
With mounting evidence from in vitro and animal studies, SSD exhibits a promising efficacy as an anti-inflammatory drug. Aqueous and ethanolic extracts of SSD show a significant effect on the inhibition of NO and TNF-α production [7]. More than dozens of bioactive compounds isolated from SSD such as (+)-epipinoresinol, (+)-pinoresinol, 2,6-dimethoxy-1,4-benzoquinone, 3-methoxydaidzein, 8-O-methylretusin, biochanin A, butesuperin A, calycosin, daidzin, formononetin, genistein, isoliquiritigenin, liquiritigenin, maackiain, odoratin, and ononin have been screened for their anti-inflammatory efficacy [37]. Furthermore, Spasuberol A, B, and C have also been identified and characterized from SSD and have been reported as anti-inflammatory agents, which was determined by decreasing NO production in RAW264.7 macrophages stimulated by LPS [38].3.4. Neuroprotective Activity
Apoptotic cell death in neuronal cells plays a key role in controlling several neurological disorders, viz., Parkinsonism, Alzheimer’s, Huntington’s, and ischemic stroke. Studies show SSD has therapeutic potential for neurological-disorder-related cell death or ischemic stroke [39].
3.5. Hematopoietic Activity
3.6. Antimicrobial Activity
4. Anticancer Activity of SSD
There has been evidence that SSD plays a positive role in the treatment of leiomyoma, breast cancer, glioblastoma, and leukemia. The anti-breast cancer effect of SSD has attracted the most attention among researchers. SSD has potential anticancer effects through apoptosis and pyroptosis induction, cell-cycle arrest, estrogen receptor hypoactivity, proteasome inhibition, anti-mutation, and ROS regulation [4][15][47][48][49][50][51]. Surprisingly, unlike anti-glioblastoma, SSD’s anti-breast cancer and anti-myeloma efficacy are reliant on ROS induction. An earlier clinical cohort study demonstrated that SSD has prospective benefits for patients with acute myeloid leukemia [52]. The chloroform and ethyl acetate subfractions of SSD administration were reported to be potent by preventing leiomyoma and reducing the expression level of TGF-beta receptor 2 [53]. A dose-dependent manner of SSD treatment exerted cytotoxicity in the myeloid-originated hematological cancer cell lines U266 and U937, which upregulated apoptosis-related proteins (PARP, procaspase-3, and Bax) and ER stress-related proteins (p-ATF2 and CHOP). Furthermore, SSD inhibited onco-miRNA (miR657) targeting the ER stress signal pathway [47]. Interestingly, KEGG and GO analysis interpreted that SSD could attenuate metastasis in the lung primarily by mediating oxidative stress, AGE-RAGE signaling, and microRNAs [54][55]. Similarly, Network pharmacology analysis revealed that SSD exhibited an anti-ovarian cancer efficacy by activating the key proteins GSK-3β, Bcl-2, and Bax [56].
Previously, studies found that EGCG, a flavanol in SSD, promotes apoptosis in the head, neck, and colorectal cancer [57][58]. Furthermore, the anticancer effect of gallic acid obtained in SSD was present in lung and prostate cancer [59][60]. Moreover, a lab previously reported a novel function of isoliquiritigenin, the active principle from SSD, as a natural inhibitor of autophagy-related miR-25 that promoted autophagy, chemosensitization, and cell-cycle arrest in drug-resistant MCF-7/ADR BC cells. Thus, isoliquiritigenin acts as a natural autophagy inducer to enhance BC chemosensitivity by targeting ULK1 [61].
The chemical constituents of SSD have massive pharmacological effects and clinical applications. SSD contains the analogue of isoliquiritigenin that is used for the prevention of BC [62]. Cyclization of OH-2′ chalcones or α, β-unsaturated isoliquiritigenin significantly induced cytotoxicity in BC cell lines. Likewise, 3′,4′,5′,4′′-tetramethoxychalcone is also known as a promising cytotoxic analogue against BC [62]. Interestingly, 3S)-7-hydroxy-8,2′,4′-trimethoxyisoflavane and (3S)-7-hydroxy-8,2′-dimethoxy-4′,5′-methylenedioxyisoflavane in SSD inhibit cell growth and the viability of MCF-7 cells. Also, sativan extracted from SSD is highly hazardous to both MCF-7, 4T1, and MDA-MB-231 cell lines, indicating that SSD is a potential candidate for the inhibition of TNBC cells [62]. Moreover, sativan, another active compound extracted from SSD, has been described to improve the expression of Bax and reduce the protein expressions of Bcl-2 and PD-L1, inhibiting the invasion and migration of TNBC cells by the upregulation of miR-200c [63].
Methanolic extracts of SSD contain several bioactive compounds, including isoliquiritigenin, genistein, 7-hydroxyflavanone, liquiritigenin, daidzein, medicarpin, and formononetin, which have significant inhibitory activity on the human 20S proteasome [37]. Various proteasome inhibitors exert anti-cancer activity in vivo and significantly induce apoptosis in cancer cells in vitro [21][24][64], and SSD and its active compounds are potential proteasome inhibitors [37]. The prevention of N-nitrosamine-induced DNA damage is a key factor for cancer chemoprevention. It has been reported that SSD containing the bioactive compounds genistein, isoliquiritigenin, medicarpin, and naringenin has antigenotoxic effects by preventing the production of the carcinogenic N-methyl-N-nitrosourea [65]. It seems that flavonoids have hydroxyl radical-scavenging capacity that is connected to their antimutagenic properties [65]. 7,4′-dihydroxy-8,2′,3′-trimethoxyisoflavan extracted from SSD has significant cytotoxicity in preventing human cancer cell lines, viz., HL-60, SMMC-7721, SW-480 MCF-7, and A-549 [66]. The ethanolic extract of SSD contains four novel isoflavones, which exert potential cytotoxicity activities in MCF-7 and MDA-MB-231 BC cell lines [49].
5. Mechanism of Anticancer Activity of SSD
5.1. RAS/Raf/MAPK Signaling
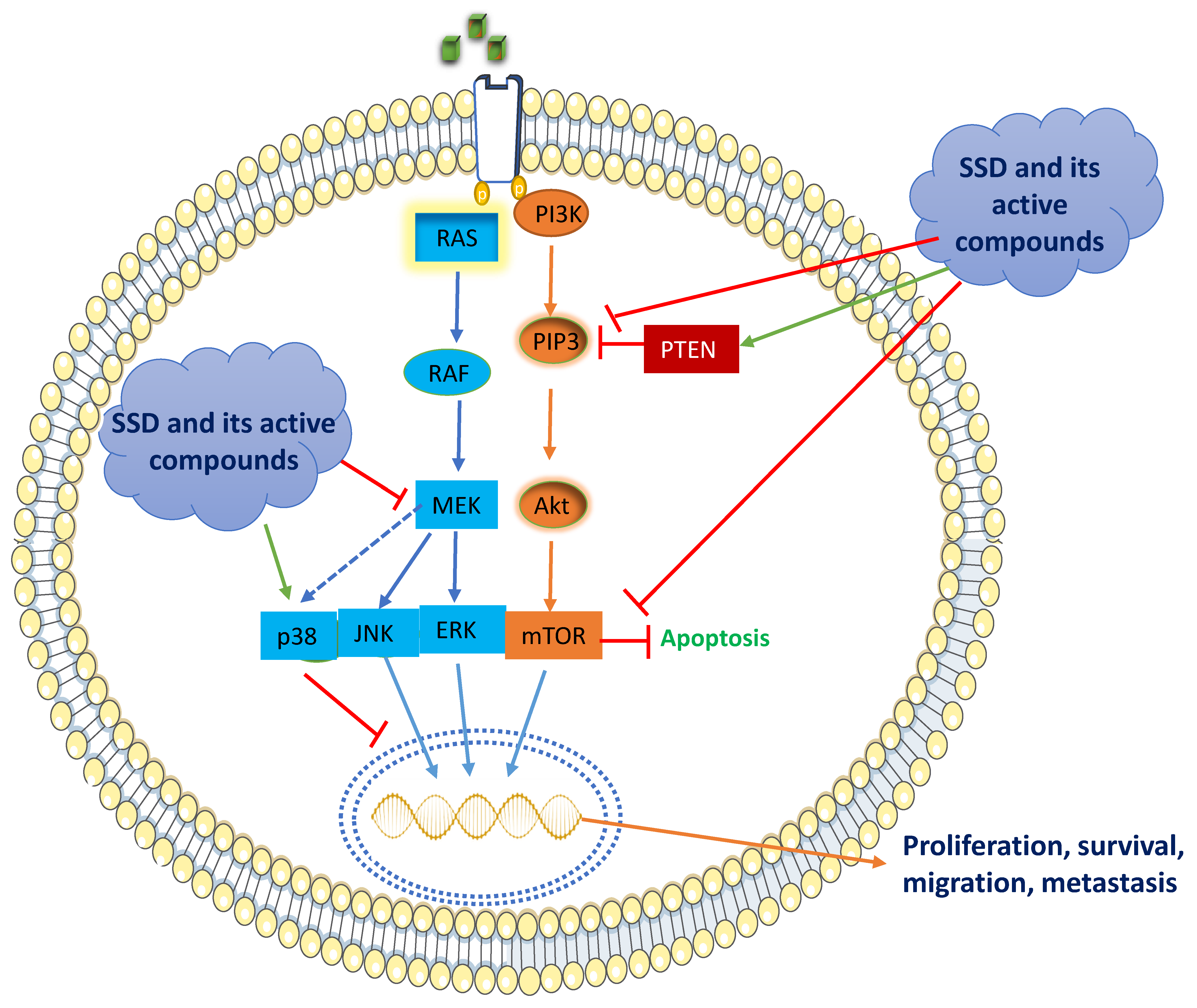
5.2. PI3K/Akt/mTOR Signaling
The remarkable downstream signaling of PI3K/Akt/mTOR is frequently engaged in most of the BC types, accounting for over 70% [70][71][72][73]. In turn, PI3K stimulates the conversion of PIP2 to PIP3, which phosphorylates protein kinase B, Akt, which in turn activates the serine/threonine kinase, mTOR [74][75]. The cascade signaling of PI3K/Akt/mTOR is a crucial key step in the cell cycle, tumor development, and survival [71]. However, the dephosphorylation of PIP3 to PIP2 activates Akt by an enzyme, PTEN, which is a well-recognized tumor-suppressor protein. (Figure 2) [72]. Activation of the PI3K/Akt pathway occurs via insulin-like growth factor 1 receptor (IGF-1R), thereby phosphorylating IGF-1R and resulting in the recruitment of Akt [76].
SSD and its active constituents exert their anti-cancer effects by networking with complex transcription factors, PI3K/Akt/mTOR. SSD comprises calycosin daidzein, formononetin, and genistein with a dose range of 80–320 μg/mL that inhibits the protein expressions of PI3K/Akt/mTOR in MCF-7 cells [50]. Resveratrol and genistein in the doses of 0.1, 1, 5, 10, 100, and 1000 nM significantly elevated PTEN expression (PI3K/Akt inhibitor) in MCF-7 and MDA-MB-435 BC cells [77]. The treatment of calycosin (25–100 μM) and formononetin (20–80 μM) inhibited PI3K/Akt signaling in T47D and MCF-7 cells by reducing the activation of IGF-1R protein levels and resulting in the inhibition of Akt phosphorylation. Similarly, the treatment of formononetin (10–40 μM) decreased the expression of p-PI3K/p-Akt in TNBC cells [78]. Another investigation demonstrated that an analog of resveratrol, MR-3 (10, 20 μM), attenuated PI3K/Akt signaling in MCF-7 cells by preventing the phosphorylation of Akt and inhibition of GSK-3β [79]. SSD contains another bioactive compound, baicalein, which is demonstrated to have an anti-BC effect through the downregulation of the expression of p-AKT, p-mTOR, NF-κB, and p-IκB and the upregulation of IκB in MCF-7 and MDA-MB-231 cells [80]. Altogether, SSD and its active constituents inhibit the PI3K/Akt pathway by inhibiting IGF-1R, phosphorylating Akt, and enhancing the inhibitory activity of PTEN.6. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/cells11182885
References
- Pang, J.; Guo, J.P.; Jin, M.; Chen, Z.Q.; Wang, X.W.; Li, J.W. Antiviral effects of aqueous extract from Spatholobus suberectus Dunn. against coxsackievirus B3 in mice. Chin J Integr Med 2011, 17, 764-769, doi:10.1007/s11655-011-0642-1.
- Qin, S.; Wu, L.; Wei, K.; Liang, Y.; Song, Z.; Zhou, X.; Wang, S.; Li, M.; Wu, Q.; Zhang, K.; et al. A draft genome for Spatholobus suberectus. Scientific Data 2019, 6, 113, doi:10.1038/s41597-019-0110-x.
- Zhang, Z.-X.; Zhang, J.; Gong, Y.; Liu, G.-Z.; Gao, L.-L.; Zhang, P.; Cai, C.-T. Morphological and physiological responses of Spatholobus suberectus Dunn to nitrogen and water availability. Photosynthetica 2019, 57, 1130-1141, doi:10.32615/ps.2019.125.
- Zhang, F.; Liu, Q.; Ganesan, K.; Kewu, Z.; Shen, J.; Gang, F.; Luo, X.; Chen, J. The Antitriple Negative Breast cancer Efficacy of Spatholobus suberectus Dunn on ROS-Induced Noncanonical Inflammasome Pyroptotic Pathway. Oxid Med Cell Longev 2021, 2021, 5187569, doi:10.1155/2021/5187569.
- Chang, C.L.; Lin, C.S.; Lai, G.H. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid. Based Complement. Alternat. Med. 2012, 2012, 984295, doi:10.1155/2012/984295.
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353-359, doi:10.1016/j.jep.2006.01.010.
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Munch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173, doi:10.1186/1472-6882-12-173.
- Tang, R.N.; Qu, X.B.; Guan, S.H.; Xu, P.P.; Shi, Y.Y.; Guo, D.A. Chemical constituents of Spatholobus suberectus. Chin J Nat Med 2012, 10, 32-35, doi:10.1016/s1875-5364(12)60007-7.
- Cui, Y.J.; Liu, P.; Chen, R.Y. [Studies on the chemical constituents of Spatholobus suberectus Dunn]. Yao Xue Xue Bao 2002, 37, 784-787.
- Cheng, J.; Liang, H.; Wang, Y.; Zhao, Y.Y. [Studies on the constituents from the stems of Spatholobus suberectus]. Zhongguo Zhong Yao Za Zhi 2003, 28, 1153-1155.
- Cui, Y.J.; Liu, P.; Chen, R.Y. [Studies on the active constituents in vine stem of Spatholobus suberectus]. Zhongguo Zhong Yao Za Zhi 2005, 30, 121-123.
- Wang, H.; Liu, Y.; Zeng, Z.; He, W. [Study on HPLC chromatographic fingerprint of anti-tumor active site SSCE of Caulis spatholobi]. Zhongguo Zhong Yao Za Zhi 2011, 36, 2525-2529.
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT - Food Science and Technology 2010, 43, 181-185, doi:10.1016/j.lwt.2009.06.020.
- Zhang, L.L.; Wang, Y.M.; Xu, M.; Wu, D.M.; Chen, J.H.; Yan, X.P. Antioxidant Activity, Phenol and Flavonoid Contents of Fourteen Mulberry Varieties Leaves. Advanced Materials Research 2013, 781-784, 1454-1459, doi:10.4028/www.scientific.net/AMR.781-784.1454.
- Liu, B.; Liu, J.; Chen, J.; Zhu, D.; Zhou, H.; Wang, X. A study on anticancer activity of Caulis Spatholobi extract on human osteosarcoma Saos-2 cells. Afr J Tradit Complement Altern Med 2013, 10, 256-260, doi:10.4314/ajtcam.v10i5.6.
- CHENG, Y.; FU, Y.; WANG, Z.; YANG, D.; CHEN, J.; WANG, D. Determination on the Contents of Condensed Tannins in Spatholobus suberectus Dunn. Extracts and Primary Study on their Anti-tumor Activities. Acta Scientiarum Naturalium Universitatis Sunyatseni 2011, 50, 75-80.
- Liu, X.Y.; Zhang, L.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Zhang, P.; Zhao, W.; Peng, K.F.; Gong, Y.; Liu, N.F. Simultaneous detection and quantification of 57 compounds in Spatholobi Caulis applying ultra-fast liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2020, 43, 4247-4262, doi:10.1002/jssc.202000496.
- Zhang, Y.; Guo, L.; Duan, L.; Dong, X.; Zhou, P.; Liu, E.H.; Li, P. Simultaneous determination of 16 phenolic constituents in Spatholobi Caulis by high performance liquid chromatography/electrospray ionization triple quadrupole mass spectrometry. J Pharm Biomed Anal 2015, 102, 110-118, doi:10.1016/j.jpba.2014.09.006.
- Li, Y.; Song, T.; Jin, X. UPLC-MS/MS assay for simultaneous determination of four compounds in rat plasma: application to pharmacokinetic study after oral administration of Caulis Spatholobi extract. Biomed Chromatogr 2016, 30, 1714-1720, doi:10.1002/bmc.3744.
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. International Journal of Molecular Sciences 2017, 18, 2390, doi:10.3390/ijms18112390.
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: a critical review. Annals of the New York Academy of Sciences 2017, 1401, 102-113, doi:10.1111/nyas.13446.
- Islam, T.; Ganesan, K.; Xu, B. New Insight into Mycochemical Profiles and Antioxidant Potential of Edible and Medicinal Mushrooms: A Review. International Journal of Medicinal Mushrooms 2019, 21, 237-251, doi:10.1615/intjmedmushrooms.2019030079.
- Toyama, T.; Wada-Takahashi, S.; Takamichi, M.; Watanabe, K.; Yoshida, A.; Yoshino, F.; Miyamoto, C.; Maehata, Y.; Sugiyama, S.; Takahashi, S.S.; et al. Reactive oxygen species scavenging activity of Jixueteng evaluated by electron spin resonance (ESR) and photon emission. Nat Prod Commun 2014, 9, 1755-1759.
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int J Mol Sci 2017, 19, doi:10.3390/ijms19010013.
- Liao, H.; Banbury, L.K.; Leach, D.N. Antioxidant activity of 45 Chinese herbs and the relationship with their TCM characteristics. Evid. Based Complement. Alternat. Med. 2008, 5, 429-434, doi:10.1093/ecam/nem054.
- Fu, Y.F.; Jiang, L.H.; Zhao, W.D.; Xi-Nan, M.; Huang, S.Q.; Yang, J.; Hu, T.J.; Chen, H.L. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci Rep 2017, 7, 8676, doi:10.1038/s41598-017-09340-9.
- Chen, H.L.; Yang, J.; Fu, Y.F.; Meng, X.N.; Zhao, W.D.; Hu, T.J. Effect of total flavonoids of Spatholobus suberectus Dunn on PCV2 induced oxidative stress in RAW264.7 cells. BMC Complement Altern Med 2017, 17, 244, doi:10.1186/s12906-017-1764-6.
- Kim, H.; Yi, S.S.; Lee, H.K.; Heo, T.H.; Park, S.K.; Jun, H.S.; Song, K.D.; Kim, S.J. Antiproliferative Effect of Vine Stem Extract from Spatholobus Suberectus Dunn on Rat C6 Glioma Cells Through Regulation of ROS, Mitochondrial Depolarization, and P21 Protein Expression. Nutr Cancer 2018, 70, 605-619, doi:10.1080/01635581.2018.1460673.
- Islam, T.; Ganesan, K.; Xu, B. Insights into health-promoting effects of Jew's ear (Auricularia auricula-judae). Trends in Food Science & Technology 2021, 114, 552-569, doi:https://doi.org/10.1016/j.tifs.2021.06.017.
- Ganesan, K.; Quiles, J.L.; Daglia, M.; Xiao, J.; Xu, B. Dietary phytochemicals modulate intestinal epithelial barrier dysfunction and autoimmune diseases. Food Frontiers 2021, 2, 357-382, doi:https://doi.org/10.1002/fft2.102.
- Ganesan, K.; Ramkumar, K.M.; Xu, B. Vitexin restores pancreatic β-cell function and insulin signaling through Nrf2 and NF-κB signaling pathways. European Journal of Pharmacology 2020, 888, 173606, doi:https://doi.org/10.1016/j.ejphar.2020.173606.
- Zhao, P.; Alam, M.B.; Lee, S.H.; Kim, Y.J.; Lee, S.; An, H.; Choi, H.J.; Son, H.U.; Park, C.H.; Kim, H.H.; et al. Spatholobus suberectus Exhibits Antidiabetic Activity In Vitro and In Vivo through Activation of AKT-AMPK Pathway. Evid Based Complement Alternat Med 2017, 2017, 6091923, doi:10.1155/2017/6091923.
- Do, M.H.; Hur, J.; Choi, J.; Kim, Y.; Park, H.Y.; Ha, S.K. Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice. Int J Mol Sci 2018, 19, doi:10.3390/ijms19092774.
- Xu, B.; Ganesan, K.; Mickymaray, S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Aboody, M.S.A. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioactive Compounds in Health and Disease 2020, 3, 15, doi:10.31989/bchd.v3i2.687.
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int J Mol Sci 2020, 21, doi:10.3390/ijms21113976.
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting Programmed Fusobacterium nucleatum Fap2 for Colorectal Cancer Therapy. Cancers 2019, 11, doi:10.3390/cancers11101592.
- Shim, S.H. 20S proteasome inhibitory activity of flavonoids isolated from Spatholobus suberectus. Phytother. Res. 2011, 25, 615-618, doi:10.1002/ptr.3342.
- Liu, X.Y.; Zhang, Y.B.; Yang, X.W.; Yang, Y.F.; Xu, W.; Zhao, W.; Peng, K.F.; Gong, Y.; Liu, N.F.; Zhang, P. Anti-Inflammatory Activity of Some Characteristic Constituents from the Vine Stems of Spatholobus suberectus. Molecules 2019, 24, doi:10.3390/molecules24203750.
- Park, H.R.; Lee, H.; Lee, J.J.; Yim, N.H.; Gu, M.J.; Ma, J.Y. Protective Effects of Spatholobi Caulis Extract on Neuronal Damage and Focal Ischemic Stroke/Reperfusion Injury. Mol Neurobiol 2018, 55, 4650-4666, doi:10.1007/s12035-017-0652-x.
- Liu, P.; Wang, D.X.; Chen, R.Y.; Chen, M.L.; Yin, J.F.; Chen, G.Y. [Effect of catechin on bone marrow cell cycle and gene expression of hematopoietic growth factors]. Yao Xue Xue Bao 2004, 39, 424-428.
- Chang, J.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y.; Pan, X.; Zhou, Y.; Lai, M.; Bian, G.; Zhou, Q.; et al. Establishment of an in vitro system based on AGM-S3 co-culture for screening traditional herbal medicines that stimulate hematopoiesis. J. Ethnopharmacol. 2019, 240, 111938, doi:10.1016/j.jep.2019.111938.
- Su, E.Y.; Chen, H.S. [Clinical observation on aplastic anemia treated by Spatholobus suberectus Composita]. Zhongguo Zhong Xi Yi Jie He Za Zhi 1997, 17, 213-215.
- Su, E.Y.; Fang, Y.H.; Chen, H.S. [Clinical observation of treating 62 patients with severe aplastic anemia failing in immunosuppressive therapy by integrative medicine]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2012, 32, 1616-1620.
- Lam, T.L.; Lam, M.L.; Au, T.K.; Ip, D.T.; Ng, T.B.; Fong, W.P.; Wan, D.C. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000, 67, 2889-2896, doi:10.1016/s0024-3205(00)00864-x.
- Guo, J.P.; Pang, J.; Wang, X.W.; Shen, Z.Q.; Jin, M.; Li, J.W. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006, 12, 4078-4081, doi:10.3748/wjg.v12.i25.4078.
- Kim, G.; Gan, R.Y.; Zhang, D.; Farha, A.K.; Habimana, O.; Mavumengwana, V.; Li, H.B.; Wang, X.H.; Corke, H. Large-Scale Screening of 239 Traditional Chinese Medicinal Plant Extracts for Their Antibacterial Activities against Multidrug-Resistant Staphylococcus aureus and Cytotoxic Activities. Pathogens 2020, 9, doi:10.3390/pathogens9030185.
- Lim, H.J.; Park, M.N.; Kim, C.; Kang, B.; Song, H.S.; Lee, H.; Kim, S.H.; Shim, B.S.; Kim, B. MiR-657/ATF2 Signaling Pathway Has a Critical Role in Spatholobus suberectus Dunn Extract-Induced Apoptosis in U266 and U937 Cells. Cancers (Basel) 2019, 11, doi:10.3390/cancers11020150.
- Lu, D.; He, H.; Wu, B.; Yao, S. Cytotoxic effect on cancer cells and structural identification of phenols from Spatholobi caulis by HPLC-ESI-MS(n). Nat Prod Commun 2009, 4, 809-812.
- Peng, F.; Zhu, H.; Meng, C.W.; Ren, Y.R.; Dai, O.; Xiong, L. New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines. Molecules 2019, 24, doi:10.3390/molecules24183218.
- Sun, J.Q.; Zhang, G.L.; Zhang, Y.; Nan, N.; Sun, X.; Yu, M.W.; Wang, H.; Li, J.P.; Wang, X.M. Spatholobus suberectus Column Extract Inhibits Estrogen Receptor Positive Breast Cancer via Suppressing ER MAPK PI3K/AKT Pathway. Evid Based Complement Alternat Med 2016, 2016, 2934340, doi:10.1155/2016/2934340.
- Wang, Z.Y.; Wang, D.M.; Loo, T.Y.; Cheng, Y.; Chen, L.L.; Shen, J.G.; Yang, D.P.; Chow, L.W.; Guan, X.Y.; Chen, J.P. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J Ethnopharmacol 2011, 133, 751-758, doi:10.1016/j.jep.2010.11.004.
- Fleischer, T.; Chang, T.T.; Chiang, J.H.; Sun, M.F.; Yen, H.R. Improved Survival With Integration of Chinese Herbal Medicine Therapy in Patients With Acute Myeloid Leukemia: A Nationwide Population-Based Cohort Study. Integr. Cancer Ther. 2017, 16, 156-164, doi:10.1177/1534735416664171.
- Bajracharya, P.; Lee, E.J.; Lee, D.M.; Shim, S.H.; Kim, K.J.; Lee, S.H.; Bae, J.J.; Chun, S.S.; Lee, T.K.; Kwon, S.H.; et al. Effect of different ingredients in traditional Korean medicine for human uterine leiomyoma on normal myometrial and leiomyomal smooth muscle cell proliferation. Arch. Pharm. Res. 2009, 32, 1555-1563, doi:10.1007/s12272-009-2107-z.
- Xie, F.; Wang, M.; Su, Y.; Xiao, K.; Chu, X.; Long, S.; Li, L.; Zhang, X.; Xue, P.; Zhu, S. Unveiling Potential Mechanisms of Spatholobi Caulis against Lung Metastasis of Malignant Tumor by Network Pharmacology and Molecular Docking. Evid Based Complement Alternat Med 2022, 2022, 1620539, doi:10.1155/2022/1620539.
- Chen, K.; Jin, C.; Cheng, Y.Y.; Zhang, Q.X.; Li, X.X.; Zhang, L. [Molecular mechanism of Spatholobi Caulis in treatment of lung cancer based on network pharmacology and molecular docking]. Zhongguo Zhong Yao Za Zhi 2021, 46, 837-844, doi:10.19540/j.cnki.cjcmm.20201118.401.
- Zhu, S.C.; Cai, J.; Wu, C.Y.; Cheng, C.S. [Molecular mechanism of Spatholobi Caulis in treatment of ovarian cancer based on network pharmacology and experimental verification]. Zhongguo Zhong Yao Za Zhi 2022, 47, 786-795, doi:10.19540/j.cnki.cjcmm.20211103.703.
- Baek, S.J.; Kim, J.S.; Jackson, F.R.; Eling, T.E.; McEntee, M.F.; Lee, S.H. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 2004, 25, 2425-2432, doi:10.1093/carcin/bgh255.
- Lim, Y.C.; Lee, S.H.; Song, M.H.; Yamaguchi, K.; Yoon, J.H.; Choi, E.C.; Baek, S.J. Growth inhibition and apoptosis by (-)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur J Cancer 2006, 42, 3260-3266, doi:10.1016/j.ejca.2006.07.014.
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett 2009, 286, 161-171, doi:10.1016/j.canlet.2009.05.040.
- Phung, H.M.; Lee, H.; Lee, S.; Jang, D.; Kim, C.-E.; Kang, K.S.; Seo, C.-S.; Choi, Y.-K. Analysis and Anticancer Effects of Active Compounds from Spatholobi Caulis in Human Breast Cancer Cells. Processes 2020, 8, doi:10.3390/pr8091193.
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013-7026, doi:10.18632/oncotarget.2192.
- Peng, F.; Meng, C.W.; Zhou, Q.M.; Chen, J.P.; Xiong, L. Cytotoxic Evaluation against Breast Cancer Cells of Isoliquiritigenin Analogues from Spatholobus suberectus and Their Synthetic Derivatives. J Nat Prod 2016, 79, 248-251, doi:10.1021/acs.jnatprod.5b00774.
- Peng, F.; Xiong, L.; Peng, C. (-)-Sativan Inhibits Tumor Development and Regulates miR-200c/PD-L1 in Triple Negative Breast Cancer Cells. Front Pharmacol 2020, 11, 251, doi:10.3389/fphar.2020.00251.
- Ganesan, K.; Du, B.; Chen, J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol Res 2022, 178, 105974, doi:10.1016/j.phrs.2021.105974.
- Inami, K.; Asada, Y.; Harada, T.; Okayama, Y.; Usui, N.; Mochizuki, M. Antimutagenic components in Spatholobus suberectus Dunn against N-methyl-N-nitrosourea. Genes and Environment 2019, 41, 22, doi:10.1186/s41021-019-0137-4.
- Wang, L.X.; Zheng, H.R.; Ren, F.C.; Chen, T.G.; Li, X.M.; Jiang, X.J.; Wang, F. Polysubstituted Isoflavonoids from Spatholobus suberectus, Flemingia macrophylla, and Cudrania cochinchinensis. Natural Products and Bioprospecting 2017, 7, 201-206, doi:10.1007/s13659-017-0121-2.
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets 2012, 16, 103-119, doi:10.1517/14728222.2011.645805.
- Saini, K.S.; Loi, S.; de Azambuja, E.; Metzger-Filho, O.; Saini, M.L.; Ignatiadis, M.; Dancey, J.E.; Piccart-Gebhart, M.J. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat Rev 2013, 39, 935-946, doi:10.1016/j.ctrv.2013.03.009.
- Lu, H.; Guo, Y.; Gupta, G.; Tian, X. Mitogen-Activated Protein Kinase (MAPK): New Insights in Breast Cancer. J Environ Pathol Toxicol Oncol 2019, 38, 51-59, doi:10.1615/JEnvironPatholToxicolOncol.2018028386.
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci 2014, 105, 252-257, doi:10.1111/cas.12349.
- Ponnusamy, L.; Kothandan, G.; Manoharan, R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim Biophys Acta Mol Basis Dis 2020, 1866, 165897, doi:10.1016/j.bbadis.2020.165897.
- Braal, C.L.; Hussaarts, K.; Seuren, L.; Oomen-de Hoop, E.; de Bruijn, P.; Buck, S.A.J.; Bos, M.; Thijs-Visser, M.F.; Zuetenhorst, H.J.M.; Mathijssen-van Stein, D.; et al. Influence of green tea consumption on endoxifen steady-state concentration in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat 2020, 184, 107-113, doi:10.1007/s10549-020-05829-6.
- Shan, Y.; Cheng, Y.; Zhang, Y.; Guan, F.Q.; Sun, H.; Ren, X.C.; Chen, Y.; Feng, X.; Yang, J.M. Triticuside A, a dietary flavonoid, inhibits proliferation of human breast cancer cells via inducing apoptosis. Nutr Cancer 2013, 65, 891-899, doi:10.1080/01635581.2013.802001.
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci Rep 2018, 8, 8323, doi:10.1038/s41598-018-25524-3.
- Ke, J.Y.; Banh, T.; Hsiao, Y.H.; Cole, R.M.; Straka, S.R.; Yee, L.D.; Belury, M.A. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol Nutr Food Res 2017, 61, doi:10.1002/mnfr.201600934.
- Serrano, M.L.; Sánchez-Gómez, M.; Bravo, M.M.; Yakar, S.; LeRoith, D. Differential expression of IGF-I and insulin receptor isoforms in HPV positive and negative human cervical cancer cell lines. Horm Metab Res 2008, 40, 661-667, doi:10.1055/s-0028-1082080.
- López-Knowles, E.; O'Toole, S.A.; McNeil, C.M.; Millar, E.K.A.; Qiu, M.R.; Crea, P.; Daly, R.J.; Musgrove, E.A.; Sutherland, R.L. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. International Journal of Cancer 2010, 126, 1121-1131, doi:https://doi.org/10.1002/ijc.24831.
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm Metab Res 2014, 46, 753-760, doi:10.1055/s-0034-1376977.
- Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H.; Way, T.D.; Chen, W.J. 3,5,4'-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol 2013, 272, 746-756, doi:10.1016/j.taap.2013.07.019.
- Im, N.K.; Lee, S.G.; Lee, D.S.; Park, P.H.; Lee, I.S.; Jeong, G.S. Spatholobus suberectus inhibits osteoclastogenesis and stimulates chondrogenesis. Am. J. Chin. Med. 2014, 42, 1123-1138, doi:10.1142/S0192415X14500700.
- Im, N.K.; Lee, S.G.; Lee, D.S.; Park, P.H.; Lee, I.S.; Jeong, G.S. Spatholobus suberectus inhibits osteoclastogenesis and stimulates chondrogenesis. Am. J. Chin. Med. 2014, 42, 1123-1138, doi:10.1142/S0192415X14500700.
