Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biotechnology & Applied Microbiology
Despite the discovery and development of an array of antimicrobial agents, multidrug resistance poses a major threat to public health and progressively increases mortality. The use of antimicrobial agents in combination can produce synergistic effects if each drug invades a different target or signaling pathway with a different mechanism of action. Therefore, drug combinations can achieve a higher probability and selectivity of therapeutic responses than single drugs.
- multidrug resistance
- bacterial infections
- synergistic effects
- antimicrobial agents
1. Introduction
The rapid emergence and spread of multidrug-resistant (MDR) bacteria has become a serious global public health threat [1]. Long-term exposure and increased use and abuse of antibiotics could result in bacterial tolerance, which renders them less effective or even ineffective, and the mechanism includes changing the targets of antibiotics [2]. MDR species are not only restricted to hospitals or healthcare environments; they are also found in humans, animals, plants, food, water, soil, and air. Moreover, they can be passed from person to person and between animals and persons. Antibiotic resistance is observed in various extracellular, intracellular, pathogenic, and nonpathogenic bacterial species. Among Gram-positive MDR species, Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecium, and Enterococcus faecalis are the most common. Among Gram-negative strains, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Acinetobacter baumannii are the most common MDR species [3]. However, methicillin-resistant Staphylococcus aureus causes pneumonia, bacteremia, soft tissue infections, and other fatal diseases [4]. Similarly, multiple antibiotic-resistant Acinetobacter spp. and Klebsiella spp. are the most commonly reported.
Recent studies suggest that biofilm-associated infections account for more than 65% of all infections, and antibiotics lack effectiveness against biofilm-associated bacteria [5]. Biofilms can shield bacteria from host defenses, disinfectants, antibiotics, and many antimicrobial agents. This leads to a reduced bacterial growth rate, decreased metabolic activity, and promotion of tolerance to antibiotics [6]. Moreover, the excessive use of antibiotics is often not tolerated by the host organism, whereas lower doses are ineffective. In addition, conventional antibiotics support antibiotic resistance in viable bacteria [7]. Pathogens growing in biofilms exhibit both adaptive resistance to all antimicrobial agents and the host immune system by 10- to 1000-fold compared to their free-living, planktonic counterparts [8]. Hence, there is urgent need to search for alternative, novel, efficient antimicrobial agents and more targeted treatment strategies to overcome antibiotic resistance. An alternative strategy currently in practice or under trials includes using different antimicrobial agents in combination to produce synergistic effects [9]. Combination therapy is an attractive and optional treatment because it represents potential adjuvant targets of non-overlapping signaling pathways and decreases the risk of developing cross-resistance [10].
Many plants have been used as sources of natural products to maintain good health, especially antimicrobial compounds [11]. Plants have evolved many alternative strategies against pathogens, which involve various phytochemicals, secondary metabolites, and other chemical compounds [12]. Bioactive compounds derived from plants, such as alkaloids, phenols, flavonoids, tannins, peptides, and other medicinally important compounds, are responsible for their antimicrobial ability against MDR pathogens [13]. Combining two or more plant extracts or their phytochemical components produces mutual antimicrobial enhancement, an unlimited pool of compounds, and the expansion or strengthening of their effects when combined as a multidrug [14]. Combinations of different drugs elicit several advantages over their use as individual moieties, including enhancing the effectiveness of other antimicrobial agents, reduction in dosage, fewer side effects, better synergistic effect, attack of multiple target sites, reduced risk, and exhibition of potent and rapid antibacterial effects against MDR pathogens [15]. The pharmacological effects of these combinations could be initiated by multiple mechanisms of action of herbal–herbal interactions.
Similarly, combining plant extracts or active phytochemicals with antibiotics improves their efficacy against resistant bacterial pathogens [16]. Synergism due to this combination helps minimize the minimum inhibitory concentrations (MICs) of these agents and reduces the economic cost and sensory impact [17]. Another strategic approach to combat MDR bacteria involves using essential oils (EOs) combined with conventional antibiotics or plant-derived phytochemicals. EOs have been widely used for their unique flavors; fragrances; and antibacterial, antioxidant, antifungal, anti-inflammatory, and anticarcinogenic properties [18]. Combining two or more EOs or their components or interactions between EOs and their components with antibiotics is a promising alternative strategy to increase their additive and synergistic antimicrobial effects. EOs and antibiotics, in combination, produce stronger bacterial inhibition compared to when they are individually administered because they target different pathways to create multifaceted effects against powerful bacterial defenses, consequently needing a decreased dose of each component [19]. The synergism between EOs and antibiotics may be attributed partly to the EO-induced permeabilization of the cell membrane, resulting in the immediate transport of antibiotics into the interior of the cell [20].
Antimicrobial nanomaterials represent another strategic approach to fighting MDR bacteria in clinical practice. Metal and metal oxide-based nanoparticles (NPs) have been widely investigated over the last decade, owing to their favorable chemical, physical, magnetic, electrical, thermal, optical, and biological properties [21]. Consequently, nanomaterials have emerged as new tools to combat deadly bacterial infections due to their specific features, such as size, shape, morphology, stability, and surface charge [22]. A combination of EOs and nanomaterials might establish functional materials with modified surfaces, improved inhibitory effects, and the ability to bind target microorganisms to achieve maximum synergistic performance [23]. Thus, combining nanomaterials with either EOs or plant extracts may improve their interaction with the bacterial cell membrane, thereby inducing the disruption, damage, and killing of bacteria [22].
2. Antibacterial Activities of Plant-Derived Compounds
Although different kinds of synthetic antimicrobial agents have been introduced to the market in many countries, natural medicine from plants might effectively treat certain diseases such as diarrhea, cold, labor pain, and dental diseases. Globally, approximately 60,000 plant species are used for medicinal purposes, of which approximately 28,000 are well-documented, and only 3000 are estimated to be traded internationally [24]. As a result, the search for herbal medicines with relevant biological activity has gained additional value as they are associated with fewer side effects and are much cheaper and affordable [25]. Plants usually produce two types of metabolites, primary and secondary, that can be found in extracts of their flowers, roots, leaves, bulbs, seeds, and bark (Figure 1). Primary metabolites are crucial for plant growth and development, whereas secondary metabolites are involved in plant defense, physiology, and environmental communication [26]. Secondary metabolites include many specialized and active compounds derived from primary metabolites. These compounds show promising results in controlling the development of resistance against bacterial pathogens, including MDR bacteria, and combating other bacterial infections. Plant secondary metabolites are classified into three categories on the basis of their biosynthetic origins: terpenoids, phenolics, and alkaloids [27].
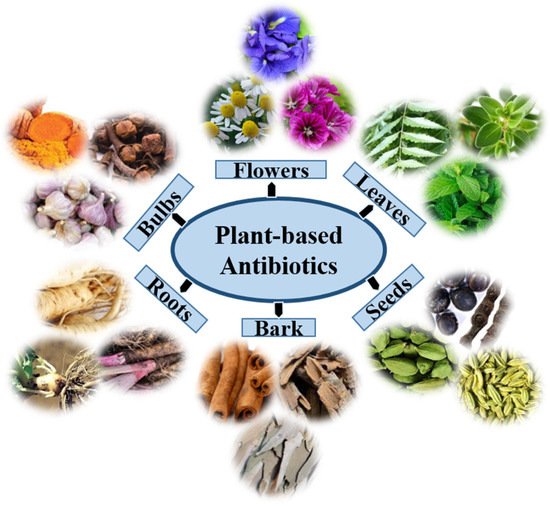
Figure 1. The extracts of plant organs, namely, the root, bark, bulbs, leaf, flower, and seed, may encompass distinctive phytochemicals with antimicrobial properties.
2.1. Terpenoids
Terpenes are an extensive and diverse group of naturally occurring, highly enriched compounds of secondary plant metabolites. On the basis of the number of their isoprene structures or units, they are classified as monoterpenes, diterpenes, triterpenes, tetraterpenes, or sesquiterpenes. Terpenes are also called isoprenoids, and their derivatives that contain additional elements, such as oxygen, are usually termed terpenoids. Monoterpenes are the smallest terpenes, comprising two isoprene units. Monoterpenes contain volatile compounds found in EOs extracted from different flowers, fruits, and leaves and are commonly used in fragrances and aromatherapy. The antimicrobial properties of these compounds have been studied for two decades, and several studies have reported that thymol, carvacrol, eugenol, and menthol exhibit significant activity against many pathogens [28]. Geraniol and thymol have shown the most activity against Enterobacter species and S. aureus and E. coli, respectively [29,30]. Diterpenes are naturally occurring chemical compounds that contain active groups such as vitamin A. Phytol is an acyclic diterpene alcohol that acts as an antitumor, cytotoxic, and anti-inflammatory agent. Diterpenes also inhibit the growth of Staphylococcus aureus, Pseudomonas aeruginosa, Vibrio cholerae, and Candida spp. [31]. Triterpenes contain six isoprene units derived from mevalonic acid and have been shown to inhibit the growth of Mycobacterium tuberculosis. The combination of rifampicin and oleanolic acid has shown synergistic antibacterial effects against some pathogens [32]. Tetraterpenes are also known as carotenoids because beta-carotene is a yellow pigment in carrots. Similarly, yellow, orange, and red organic pigments are produced by plants, and these substances have effective antifungal and antibacterial properties [33]. Sesquiterpenes are the most diverse group of terpenoids, consisting of three units of isoprene with a lower vapor pressure than monoterpenes because of their high molecular weight. Farnesol, a natural sesquiterpene, demonstrated antibacterial activity against S. aureus and S. epidermidis [34].
2.2. Phenolics
Phenols are the simplest bioactive phytochemicals. They are monomeric components of polyphenols and acids with a single substituted phenolic ring and are typically found in plant tissues such as melanin and lignin. The components catechol, orcinol, tarragon, pyrogallol, phloroglucinol, pyrocatechol, resorcinol, and thyme are effective against viruses, bacteria, and fungi [35]. Both catechol and pyrogallol are hydroxylated phenolic compounds that are toxic against microorganisms; catechol has two hydroxyl groups, while pyrogallol has three. The microbial toxicity of phenolic compounds depends mainly on the number and position of their hydroxyl groups, as hydroxylation increases toxicity [36]. The presence of two or more hydroxyl groups located at ortho, para, or meta positions to each other is the key factor for their antimicrobial activity. The presence of a hydroxyl group at the meta position of thymol makes it a more effective antibacterial agent than carvacrol, which has a similar structure, whose hydroxyl group is in the ortho position.
2.3. Alkaloids
Alkaloids are cyclic-nitrogen-containing organic compounds that have various chemical structures. More than 18,000 alkaloids have been discovered and studied phytochemically from different sources. Alkaloids are grouped into several classes, as natural, semi-synthetic, or synthetic, on the basis of their heterocyclic ring systems and biosynthetic precursors [37]. Alkaloids have various pharmacological activities, including antitumor, antihyperglycemic, anti-allergic, antidiabetic, antihyperlipidemic, and antibacterial. Piperine, berberine, quinolone, reserpine, sanguinarine, tomatidine, chanoclavine, conessine, and squalamine are the most important alkaloids with potent antibacterial activity. Piperine isolated from Piper nigrum and Piper longum inhibited the growth of mutant S. aureus when co-administered with ciprofloxacin [38].
3. Antimicrobial Efficacy of EOs
EOs are aromatic, lipophilic, and complex mixtures of volatile secondary metabolites that are mainly obtained from different parts of plants, such as leaves, herbs, flowers, buds, fruits, twigs, wood, bark, roots, and seeds [73]. EOs are extracted using hydrodistillation or steam distillation. EOs are lighter than water, with a strong flavor and odor reminiscent of their plant origin. The chemical composition of EOs is highly complex, with the main components being flavonoids, flavones, flavonols, phenols, polyphenols, tannins, alkaloids, quinones, coumarins, terpenoids, polypeptides, and lectins [22]. These compounds show potential pharmacological activities such as hepatoprotective, anti-inflammatory, antioxidant, anticancer, antiseptic, insecticidal, anti-parasitic, anti-allergic, antiviral, and antimicrobial properties [74]. Essential-oil-based products are in high demand in aromatherapy; as flavor-enhancers in food, beverages, cosmetics, perfumes, soaps, plastics, and resins; and in the pharmaceutical industries [75]. EOs have more than 50 components; however, only two or three of them are the major components present in high proportions. The other minor components are present in low amounts.
The amount of the different components of EOs varies with the parts and species of plants, as they are chemically derived from compounds and their derivatives [76]. The major constituents of EOs are terpenes and terpenoids, while other important compounds include aromatic and aliphatic constituents. The volatile components of EOs include a variety of chemicals such as alcohols (such as menthol, borneol, nerol, and linalool), acids (such as geranic acid and benzoic acid), aldehydes (such as citral), esters (such as linalyl acetate, citronellyl acetate, and menthyl), ketones (such as carvone, camphor, and pulegone), hydrocarbons (such as α-pinene, α-terpinene, myrcene, camphene, and p-cimene), ketones (such as camphor, pulegone, and carvone), phenols (such as carvacrol and thymol), lactones (such as bergapten), and peroxides (such as ascaridole), all of which play major roles in the composition of EOs (Figure 2) [77]. EOs show strong antibacterial activity against various pathogenic bacteria, including MDR pathogens, by penetrating the membrane of bacterial cells and disrupting their cellular structure. The antibacterial effectiveness of EOs differs across plant species and target bacteria depending on their cell wall structure (Gram-positive or Gram-negative). The association of some major constituents of EOs, such as eugenol, thymol, carvacrol, carvone, p-cymene, terpinene-4-ol, and cinnamic aldehyde, which easily penetrate and split in the lipid membrane, could disrupt the cell membrane; prevent cellular respiration; and lead to the loss of cell membrane integrity, removal of cellular contents, and finally cell death [78].
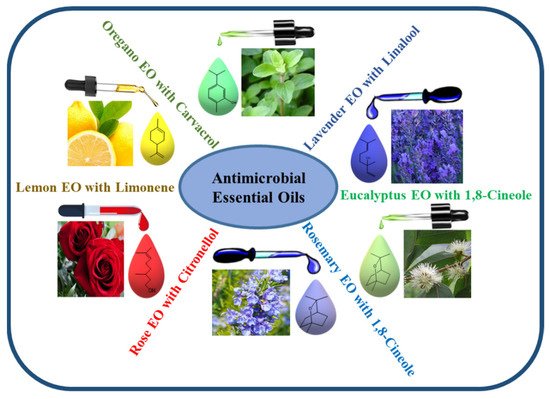
Figure 2. Schematic representation of some important EOs containing various components that have been screened for their antimicrobial properties.
EOs of Thymus serrulatus and Thymus schimperi were shown to possess strong antibacterial activity against Lactobacillus and S. mutans, with higher contents of thymol and carvacrol compounds reported to be the cause of this inhibition [79]. Similarly, EOs have been isolated from various parts of Eugenia caryophylata, such as the buds, leaves, and stems, with the main components being eugenol, β-caryophyllene, and eugenyl acetate. These EOs are effective against S. aureus, E. coli, B. subtilis, and S. typhimurium [80]. Likewise, tea tree EO has been reported to cause changes in the membrane permeability and mycelial morphology of Monilinia fructicola [81]. Furthermore, a recent study of lavender EO against A. hydrophila, A. caviae, A. dhakensis, C. freundii, P. mirabilis, and S. enterica showed the presence of the major compounds linalool and linalyl acetate [82]. In another study, winter savory EO exhibited the strongest inhibitory effect against clinical oral isolates of Candida spp., with thymol as the major compound [83]. It has been reported that among the commercially available EOs, such as anise, cinnamon, clove, cumin, laurel, Mexican lime, and Mexican oregano, oregano EO has the highest antibacterial activity against S. typhimurium and E. coli, with thymol as its major compound [84].
4. Antimicrobial Nanomaterials
With the emergence of bacterial resistance and biofilm-associated infections, clinical research is needed to develop novel, effective, long-term antibacterial and biofilm-preventative agents. Metals have been extensively studied among the most promising novel antimicrobial agents [111]. Recently, metal-based nanomaterials have become the most extensively and rapidly emerging materials in the field of medicine. Different types of metallic NPs have demonstrated strong antibacterial activity in many recent studies [112]. Generally, NPs have fascinating characteristics, such as a high surface area-to-volume ratio, size, shape, and surface activity, and exhibit superior electrical, catalytic, and optical properties. Due to their unique properties, NPs have a more well-developed surface than their microscale counterparts, affecting their antimicrobial efficiency and effectiveness [113]. Similar to antibiotics, metals can selectively inhibit metabolic pathways by interacting with bactericidal activity and ultimately kill MDR bacteria [112]; however, cells deviate from metal transport systems and metalloproteins [114]. Hence, NPs showed noticeable antimicrobial activity against both Gram-negative and Gram-positive pathogens such as E. faecalis, B. subtilis, S. epidermidis, multidrug-resistant S. aureus, and E. coli strains.
Metal NPs such as Ag, Au, Cu, Zn, Ti, Ga, Al, and Pt [115] and metal oxide NPs such as CuO, MgO, ZnO, TiO2, NiO2, SiO2, and Fe3O4 are known to display various antimicrobial properties, which have been known and applied for decades [116]. In addition, graphene oxide (GO) and carbon nanotubes (CNTs), such as single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), are also excellent candidates due to their antimicrobial activities (Figure 3). Recently, metal–organic frameworks (MOF) and metal sulfide nanomaterials, such as FeS-, Ags-, ZnS-, and CdS-MOFs and Zn-, Cu-, and Mn-based MOFs, have also been proven to have antibacterial activities [117]. Multimetallic NPs, particularly NPs formed by at least two metals, such as bimetallic, trimetallic, and quadrametallic NPs, display rich optical, electronic, and magnetic properties. The properties of multimetallic NPs, including size, shape, surface area, and zeta potential, enhance their interaction with bacterial cell membranes. They could disrupt cell membranes, produce reactive oxygen species (ROS), damage the DNA, induce protein dysfunction, and may be potentiated by the host immune system [23].
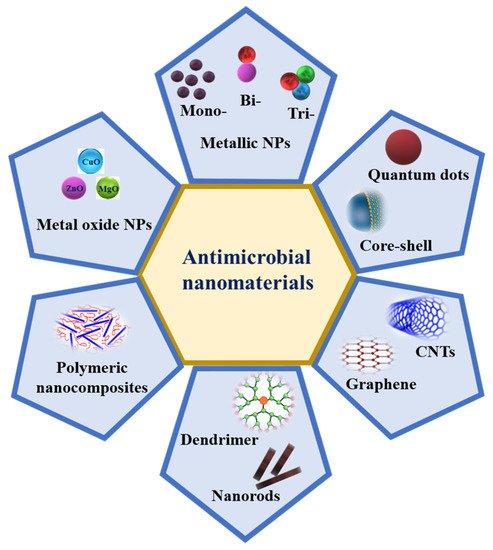
Figure 3. Schematic representation of different nanomaterials that possess antimicrobial activity.
Metallic biopolymer-based nanocomposite systems are well-known candidates as antimicrobial nanomaterials. In particular, cationic chitosan-based NPs bind to anionic cell membranes, resulting in alterations to the cell membrane, leakage of intracellular compounds, and eventually cell death [118]. Antimicrobial peptides (AMPs) have attracted great interest because of their high biocompatibility and low probability of inducing bacterial resistance. AMP-conjugated nanomaterials can hinder the growth of pathogens and kill bacteria on the basis of the inherent action of typical combination strategies [119].
AgNPs are considered the most common antimicrobial agents that can destroy a wide range of Gram-negative and Gram-positive bacteria. Ag ions combine with disulfide or sulfhydryl groups of enzymes, disrupt normal metabolic processes, and ultimately lead to cell death [120]. The bactericidal efficacy of Au NPs might have a greater chance of penetrating the bacterial cell wall by generating holes, leading to increased permeability and higher oxidative stress within the cytoplasm. Similarly, ZnO NPs displayed vigorous antimicrobial activity by releasing Zn2+ ions and generating ROS, owing to their electrostatic interaction and internalization. In contrast, smaller ZnO NPs increased the interaction and abrasiveness of the bacterial cell wall [121]. Cu and CuO NPs showed excellent antimicrobial activity against different strains of bacteria by releasing Cu2+ ions and stimulating ROS production [122]. Various studies have revealed the visible-light-induced antibacterial properties of Fe-, Cu-, Ni-, and Ag-doped TiO2 NPs against E. coli and S. aureus [123,124]; however, TiO2 NPs adversely affect human cells and tissues, so their use remains limited. SiO2 NPs, especially mesoporous NPs, have attracted considerable attention because their properties, such as size, matrix, and surface functions, which can be tuned to improve their interaction with and penetration of biofilm-producing bacteria [125].
Compared to monometallic NPs, multimetallic NPs, such as bi-, tri-, and quadrametallic NPs, have gained great importance and interest due to their unique physical, chemical, electrical, optical, and catalytic properties and applications in different fields [126]. Multimetallic NPs can be altered or tuned by controlling their structure, morphology, and chemical composition to achieve strong synergistic interactions and performance [127]. When at least two metals are formed as NPs, combinatorial approaches, such as structural changes, deduction of the lattice parameters, and total electronic charge shift improvements, are expected [128]. Recently, bimetallic Ag/Cu and Cu/Zn [129], and trimetallic Cu/Cr/Ni [130] and CuO/NiO/ZnO [131] NPs have exhibited remarkably improved antimicrobial performance compared to monometallic NPs.
Core–shell quantum dots (CSQDs) are a new type of fluorescent antibacterial nanomaterial with unique physical and chemical properties. Owing to their high electron transfer, CSQDs produce a large number of free electrons and holes that accumulate ROS inside the cell, inhibiting their respiration and replication [167]. CSQDs exert antimicrobial effects by destroying cell walls, binding with genetic material, and inhibiting energy production.
Additionally, the antibacterial properties of graphene involve both chemical and physical modes of action. The chemical action is associated with oxidative stress generated by charge transfer and ROS, while the physical action is induced by the direct contact of graphene with bacterial membranes [175]. Similarly, CNTs are more effective and cost-efficient, exhibiting strong antimicrobial properties owing to their remarkable structure. This mechanism is based on the interaction of CNTs with microorganisms and the disruption of their metabolic processes, cellular membranes, and morphology.
Dendrimers are macromolecules with highly branched tree-like dendritic structures, narrow sizes, relatively large molecular masses, and well-defined globular structures [183]. Dendrimers peripherally cationic and highly water soluble due to numerous peripheral hydrophilic groups compatible with water [184]. Dendrimers can incorporate biologically active agents in the interior or periphery; therefore, they serve as carriers of biologically active agents [185]. Antimicrobial polymers or their composites can prevent or suppress the growth of microbes on their surfaces or in the environment. Positively charged polymer surface groups are attracted to negatively charged cell membranes, leading to cell membrane damage and cell death [186].
5. Synergistic Antimicrobial Activity of Plant Extracts, EOs, and Nanomaterials
A synergistic effect is a process in which chemical substances or biological structures interact or combine to create an effect greater than the sum of the effects of the individual components. Synergy is the concept wherein the performance of two or more antimicrobial agents is combined and the effects of such mixtures are greater than those of the separate or individual components, enhancing solubility. In recent years, the synergistic combination of different antimicrobials and plant extracts has been considered a unique strategy to increase the spectrum of antimicrobial activity of these substances and prevent the development of resistant strains [194]. Plant extracts, EOs, and nanomaterials are commonly used as antimicrobial agents for the treatment of many infectious diseases. However, these antimicrobials are not very effective against acute infections because they lack a standardized and clinically applicable pharmaceutical form. Consequently, various antibiotics have been discovered as synthetic antimicrobials; however, these drugs are highly toxic and have poor tolerability, so bacteria develop resistance against them. Hence, a possible approach to improve and enhance antibacterial activity is to use combinations of different antimicrobials. These combinatorial approaches can be used alone or with other antimicrobials against a wide range of pathogens [195]. Compared to the individual substances or components, multicomponent antimicrobials display increased antimicrobial activity; therefore, other molecules present in the antimicrobial agents could control the function of the main components and improve their synergistic effects. Moreover, combining different antimicrobials offers many advantages, including a reduction in dosage, fewer side effects, decreased toxicity, extensive antibacterial action, and the ability to attack multiple target sites with increased efficacy [196]. Some combinations of antimicrobial agents, such as plant extracts, EOs, antibiotics, and NPs, are summarized in Table 1.
Table 1. Antimicrobial activity of combinations of plant extract, EOs, antibiotics, and NPs against different pathogens.
| Antimicrobial Agents | Combinations | Pathogens | Ref. |
|---|---|---|---|
| EOs/EOs | Melaleuca alternifolia/Cupressus sempervirens | E. coli | [195] |
| EOs/antibiotics | Eucalyptus globulus/oxacillin | S. aureus | [195] |
| EOs/NPs | Lemongrass/chitosan NP | E. coli, S. aureus | [197] |
| Plant extract/antibiotics | Salvadora persica/amoxicillin | P. gingivalis, T. forsythia | [198] |
| Plant extract/EOs | Origanum vulgare/carvacrol | S. aureus | [199] |
| Plant extract/NPs | Vatica diospyroides/Ag NPs | S. aureus, B. subtilis | [200] |
| NPs/antibiotics | AgNPs/fluconazole | S. aureus, E. coli | [201] |
| β-Lactam/β-lactamase inhibitor | amoxicillin/potassium clavulanate | S. aureus | [202] |
Combinations of antimicrobial agents, such as EOs/EOs, plant extract/plant extract, and NPs/NPs, already have confirmed antimicrobial activities [203]. Ncube et al. evaluated the bulb and leaf extracts of three medicinal plants, independently and in combination, against S. aureus. Their results showed the strongest synergistic effect compared with the effects observed with individual extracts [12]. Similarly, a combination of Bulbine frutescens and Vernonia lasiopus plant extracts showed improved antimicrobial activity against E. coli [204]. Obuekwe et al. found the largest zones of inhibition against S. aureus using a combination of Ocimum gratissimum and Ficus exasperate, and Bryophyllum pinnatum and Ocimum gratissimum against E. coli [205]. Recently, EO–EO associations showed a synergistic effect against vancomycin-resistant enterococci (VRE), methicillin-resistant S. aureus (MRSA), and extended-spectrum β-lactamase (ESBL)-producing Escherichia coli [195]. A mixture of R. abyssinicus and D. penninervium EOs showed strong synergistic effects against MRSA and P. aeruginosa [206].
The synergistic antibacterial activities of cumin, cardamom, and dill weed EOs against C. coli and C. jejuni have been reported [91]. In a previous study, a combination of cinnamon and clove EOs showed synergistic antibacterial activity against foodborne S. aureus, L. monocytogenes, S. typhimurium, and P. aeruginosa [207]. Garza-Cervantes et al. examined the synergistic antimicrobial activities of silver in combination with other transition metals (Zn, Co, Cd, Ni, and Cu). Their results exhibited synergism since the antimicrobial effects of the combinations against E. coli and B. subtilis increased up to eightfold when compared to the individual metals [208]. Similarly, β-lactam is the most common bactericidal agent recommended for the treatment of several infectious diseases. However, the increasing emergence of β-lactam resistance due to β-lactamase enzyme production is one of the most serious public health threats. Hence, current clinical trials suggest the use of proper combinations of β-lactam and β-lactamase inhibitors [209]. β-Lactam inhibitors are associated with β-lactam antibiotics because they are hydrolyzed by β-lactamases, and their main objective is to protect the associated antibiotics. β-Lactam inhibitors prevent the hydrolytic action of β-lactam antibiotics by binding to the active site of β-lactamase enzymes [210].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10092219
This entry is offline, you can click here to edit this entry!
