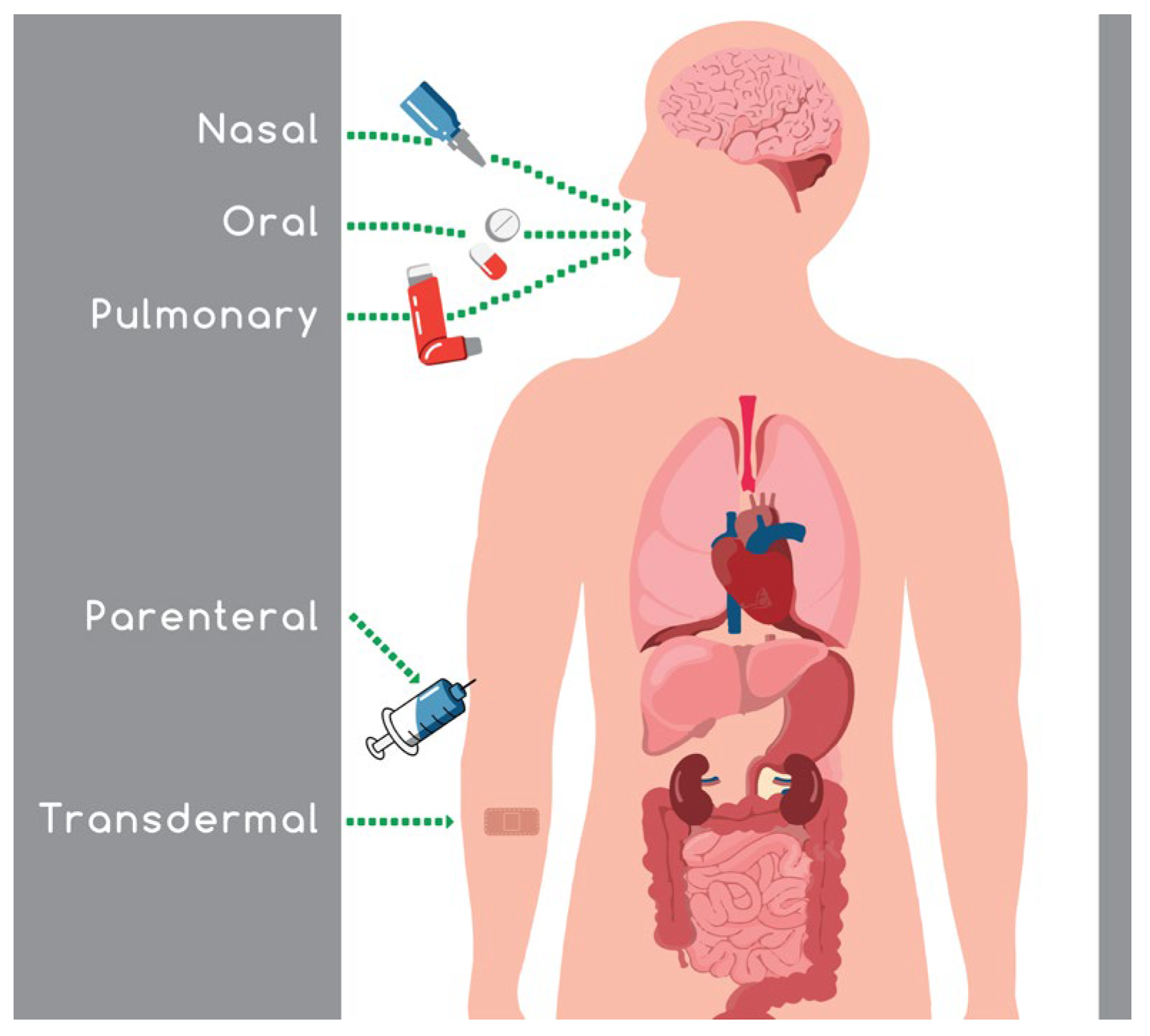
Intranasal absorption is a favored route because it avoids the gastrointestinal and hepatic metabolism, leading to an increase in drug bioavailability, and a reduction in the side effects and the required dose administered. The ongoing challenging task in the field of nasal drug delivery is the maintenance of an efficient concentration of the active substance in the target area for an adequate period of time.
- nasal drug delivery
- poloxamer
- chitosan
- gellan gum
- vaccines
- excipients
- nanoparticles
1. Introduction
|
Route (Absorption Site) |
Advantages |
Disadvantages |
Barrier Properties and Delivery Challenges |
|---|---|---|---|
|
Intravenous |
|
|
None |
|
Subcutaneous |
|
|
|
|
Inhalation (lungs) |
|
|
|
|
Oral (intestines) |
|
|
|
|
Transdermal (skin) |
|
|
|
|
Nasal (nasal mucosa surface) |
|
|
|
|
Buccal (oral mucosal surface) |
|
|
|
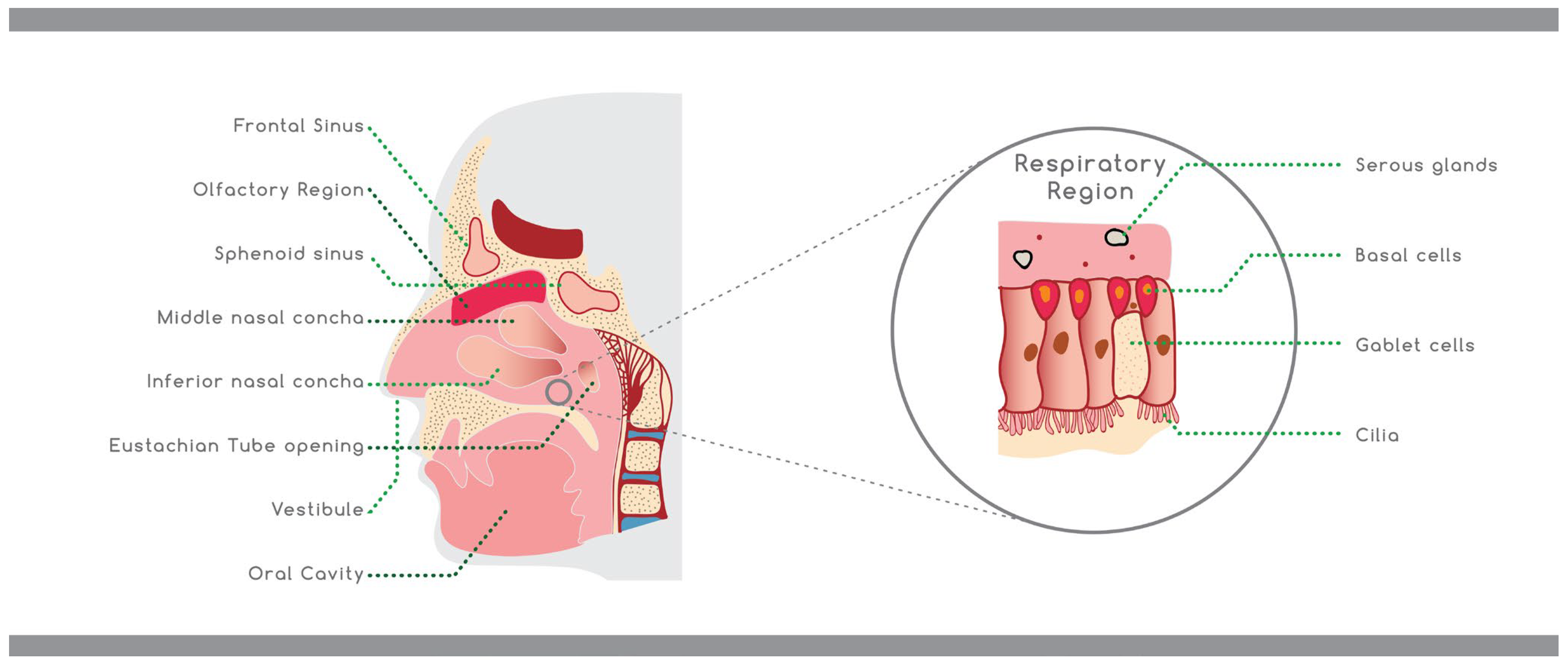
2. Anatomy and Physiology of the Nose
3. Nasal Drug Delivery
4. Factors That Affect the Nasal Drug Absorption
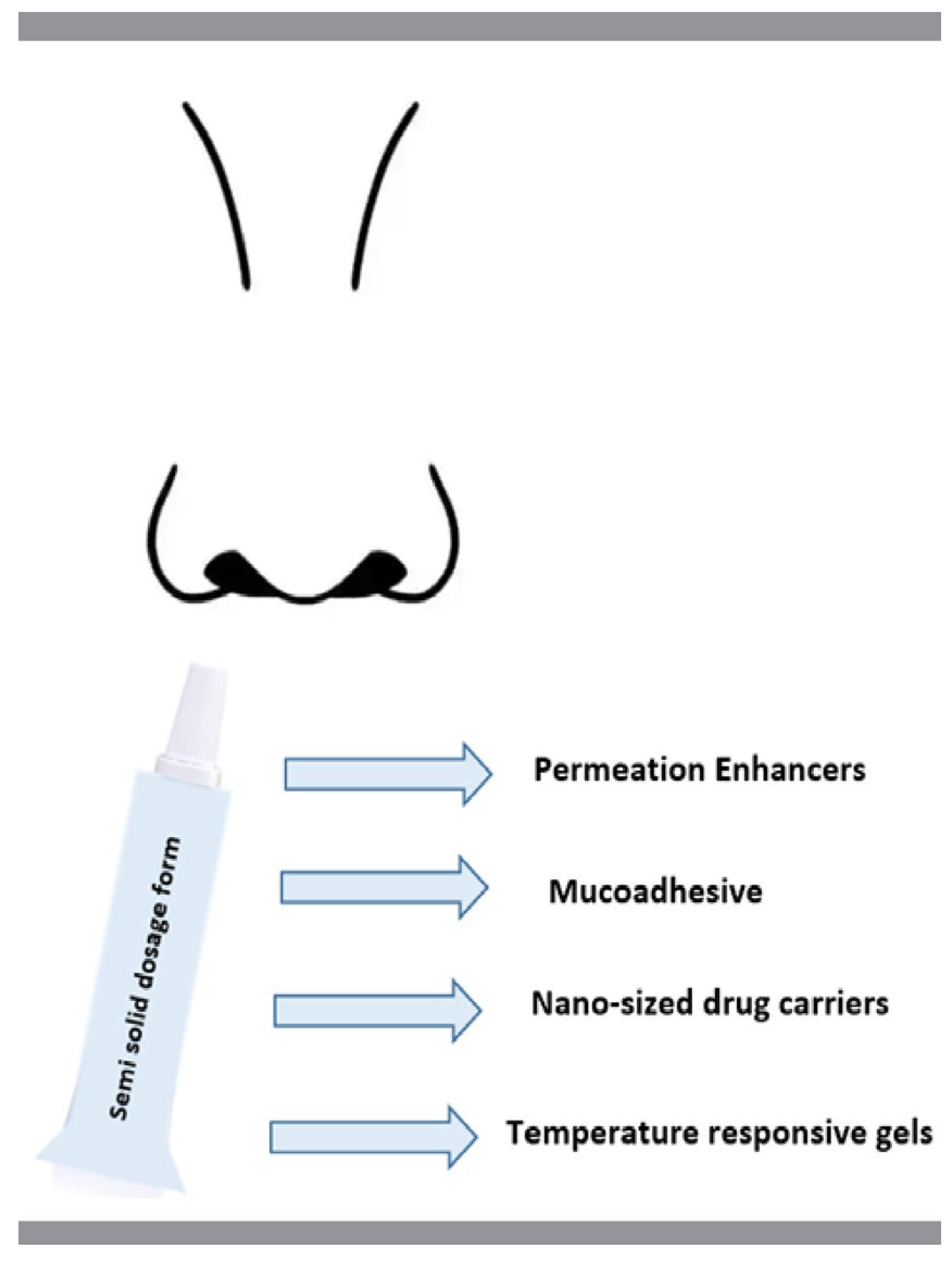
5. Excipients Used in Modified Drug Release Semi-Solid Pharmaceutical Dosage Forms for Nasal Administration
|
Nasal Dosage Form |
Drug Release Rate * |
API |
Excipients |
Refs. |
|---|---|---|---|---|
|
in situ gel |
biphasic |
huperzine A |
poloxamers (407, 188), CS, castor oil, polyoxyl 40 hydrogenated castor oil, 1,2- propanediol, Ringer’s solution |
[32] |
|
in situ gel |
biphasic |
almotriptan |
poloxamer (407, 188), Na-CMC, glyceryl behenate glyceryl palmitostearate, glyceryl monostearate, precirol |
[33] |
|
in situ gel |
biphasic |
sumatriptan |
poloxamers (407, 188), carrageenan, soybean phospholipids, cholesterol, tween 80, sodium caprate, sodium cholate, clostridium perfringens enterotoxin, sodium caprate |
[34] |
|
in situ gel |
controlled |
ziprasidone |
poloxamers (407, 188) β-cyclodextrin, HPMC E5, PEG 6000, PEG 4000, polyethylene, HPMCK4M |
[35] |
|
in situ gel |
controlled |
geniposide |
poloxamers (407, 188), HPMC, borneol, benzalkonium chloride, NaCl |
[36] |
|
in situ gel |
sustained |
rivastigmine hydrogen tartrate |
poloxamer 407, poly (lactic-co-glycolic acid), polymeric NPs |
[37] |
|
in situ gel |
sustained |
mometasone furoate |
poloxamer 407, Carbopol® 974P NF, PEG 400, NaCl, benzalkonium chloride, dexpanthenol, triethanolamine |
[38] |
|
in situ gel |
controlled |
montelukast sodium |
poloxamer 407, HPMC K4M, PEG 400, methyl paraben |
[39] |
|
in situ gel |
controlled |
hydrocortisone |
poloxamer 188, Carbopol 934, PG, benzalkonium chloride, triethanolamine, isopropyl alcohol |
[40] |
|
NP |
biphasic |
pramipexole dihydrochloride |
CS, sodium tripolyphosphate |
[41] |
|
NP |
biphasic |
efavirenz |
CS chloral hydrate, glucosamine chloral hydrate, N-acetylglucosamine, HP-β-CD, Tween 80 |
[42] |
|
NP |
controlled |
sitagliptin |
CS, glacial acetic acid, tripolyphosphate |
[43] |
|
NP |
delayed |
human serum albumin |
CS low molecular weight, acetic acid, mucin, sialic acid |
[44] |
|
in situ misemgel |
controlled |
raloxifene hydrochloride |
peppermint oil, n-propanolol, n-butanol, Tween® 80, PEG 200, PG, GG, TPGS, linoleic acid, Kolliphor®, RH 40 |
[45] |
|
in situ gel loaded NPs |
biphasic |
voriconazole |
GG, clove oil, nanotransferosomes, Tween 80, lecithin |
[46] |
|
nanoemulsion |
biphasic |
quetiapine |
Capmul MCM, Emalex LWIS 10, PEG 400, Transcutol P, Tween 80, water, Labrafil M 1944 CC, isopropyl myristate, sesame oil, Lauroglycol 90, miglyol 840 |
[47] |
|
NPs |
sustained |
dolutegravir sodium |
HP-β-CD, DPC, Tween 80, DMSO |
[48] |
|
NPs |
slow |
acetylcholinesterase reactivator |
L-α-phosphatidylcholine, 75% soybean phosphatidylcholine, dihexadecylmethylhydroxyethylammonium bromide, Tween 80, Phospholipon 80, Lipoid S75, 1-(o-tolylazo)-2-naphthol, pyrene, pyridine-2-aldoxime methochloride (Pralidoxime) |
[49] |
* Drug release rate as stated by the author(s); CS: chitosan, DPC: diphenyl carbonate, GG: gellan gum, HPMC: hydroxypropylmethylcellulose, HP-β-CD: hydroxypropyl-β-cyclodextrin, Na-CMC: sodium carboxymethylcellulose, NPs: nanoparticles, PEG: polyethylene glycol, PG: propylene glycol, TPGS: d-α-tocopheryl polyethylene glycol 1000 succinate.
6. Excipients Used in Modified Drug Release Vaccines for Nasal Administration
|
Vaccine |
Release Rate * |
API |
Excipients |
Refs. |
|---|---|---|---|---|
|
NPs |
extended |
Encephalitis-chimeric virus |
trimethyl CS, glycol CS, 6-maleimidohexanoic acid, 1-ethyl-3-(3-dimethylamino propyl)carbodiimide, N-hydroxysuccinimide, sodium tripolyphosphate, phenylmethylsulphonyl fluoride, fluorescein isothiocyanate-conjugated bovine serum albumin, bovine serum albumin, polystyrene microplates, IFN-γ, IL-4 cytokine |
[51] |
|
NPs |
slow |
plasmid DNA encoding 5p36/LACK leishmanial antigen |
CS microparticles, glyceraldehyde |
[52] |
|
NPs |
controlled |
bovine serum albumin |
aminated CS, aminated and thiolated CS, CS, N-(2-hydroxyethyl) ethylenediamine, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, thioglycolic acid, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), trypsin-EDTA |
[53] |
|
hydrogel |
prolonged |
antigen that generates nasal tissue resident memory CD8+ T cells |
CS, poloxamers (188 and 407), ovalbumin protein, lipopolysaccharide |
[54] |
|
NPs |
biphasic |
r4M2e.HSP70c antigen |
N,N,N-trimethyl CS, trimethyl CS, glycerin |
[55] |
|
NPs |
biphasic |
tetanus toxoid |
CS, NPs, paraffin oil, nanospheres |
[56] |
|
NPs |
biphasic |
tetanus toxoid |
N-trimethyl CS, CS, dextran microspheres, tripolyphosphate, lactose, Span 80, Tween 80 |
[57] |
|
NP |
gradual |
bovine serum albumin, ovalbumin, and myoglobin |
low molecular weight CS, Compound 48/80, MTT (3-[4, 5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide), albumin-fluorescein isothiocyanate conjugate (FITC-BSA), trehalose, Dulbecco’s modified Eagle medium (DMEM) and Roswell Park Memorial Institute (RPMI), Bicinchoninic acid (BCA) assay and micro BCA kits, Fetal bovine serum (FBS), wheat germ agglutinin Alexa Fluor® 350 Conjugate and Lysotracker® Red DND 99 |
[58] |
|
NPs |
extended |
PPE17 antigen (for tuberculosis) |
CS, SA |
[59] |
|
NP |
burst release prevented |
PR8 influenza virus |
SA, CS, N,N,N-trimethyl CS, concanavalin A |
[60] |
|
NPs |
biphasic |
inactivated influenza virus |
SA powder, class B CpG ODN 2007 with a phosphorothioated backbone, 2,3-bis-(2-methoxy-4-nitro-5- sulfophenyl)-2H -tetra- zolium-5-carboxanilide, Tween 80 and Span 80 |
[61] |
|
NPs |
prolonged |
bovine serum albumin |
Poly(D,L-lactide-co-glycolide), Bisphenol-A-ethoxylate di-acrylate, ethylenediamine, tetrahydrofuran, poly(vinyl alcohol) |
[62] |
|
nanogel |
gradually |
surface protein A fusion antigens |
pullulan with 1.3% cholesterol and 23% amino residues |
[63] |
|
nanogels |
complete release in 6 h |
Ovalbumin |
squalane oil, cyclohexane, surfactant sucrose laurate (L-195) |
[64] |
|
nanodispersion |
prolonged |
Ovalbumin |
Epsiliseen®-H (ϵ-polylysine), dextran sulfate sodium salt, hydrogen chloride, sodium hydroxide |
[65] |
* Drug release rate as stated by the author(s); CS: chitosan, NPs: nanoparticles, SA: sodium alginate.
This entry is adapted from the peer-reviewed paper 10.3390/ma15196547
References
- Sadeghi, S.; Lee, W.K.; Kong, S.N.; Shetty, A.; Drum, C.L. Oral administration of protein nanoparticles: An emerging route to disease treatment. Pharmacol. Res. 2020, 158, 104685.
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2018, 18, 19–40.
- Dubey, S.K.; Parab, S.; Dabholkar, N.; Agrawal, M.; Singhvi, G.; Alexander, A.; Bapat, R.A.; Kesharwani, P. Oral peptide delivery: Challenges and the way ahead. Drug Discov. Today 2021, 26, 931–950.
- Leonard, A.K.; Sileno, A.P.; Brandt, G.C.; Foerder, C.A.; Quay, S.C.; Costantino, H.R. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int. J. Pharm. 2007, 335, 138–146.
- Rapoport, A.; Winner, P. Nasal delivery of antimigraine drugs: Clinical rationale and evidence base. Headache 2006, 46, 192–201.
- Laffleur, F.; Bauer, B. Progress in nasal drug delivery systems. Int. J. Pharm. 2021, 607, 120994.
- Illum, L. Nasal drug delivery—Recent developments and future prospects. J. Control. Release 2012, 161, 254–263.
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta Biomembr. 2009, 1788, 892–910.
- Wolburg, H.; Wolburg-Buchholz, K.; Sam, H.; Horvát, S.; Deli, M.A.; Mack, A.F. Epithelial and endothelial barriers in the olfactory region of the nasal cavity of the rat. Histochem. Cell Biol. 2008, 130, 127–140.
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198.
- Pires, A.; Fortuna, A.; Alves, G.; Falcão, A. Intranasal drug delivery: How, why and what for? J. Pharm. Pharm. Sci. 2009, 12, 288–311.
- Xi, J.; Si, X.A.; Kim, J.; Zhang, Y.; Jacob, R.E.; Kabilan, S.; Corley, R.A. Anatomical Details of the Rabbit Nasal Passages and Their Implications in Breathing, Air Conditioning, and Olfaction. Anat. Rec. 2016, 299, 853–868.
- Gizurarson, S. The effect of cilia and the mucociliary clearance on successful drug delivery. Biol. Pharm. Bull. 2015, 38, 497–506.
- Ugwoke, M.I.; Verbeke, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 2010, 53, 3–21.
- Lazovic, G.D.; Daniel, R.K.; Janosevic, L.B.; Kosanovic, R.M.; Colic, M.M.; Kosins, A.M. Rhinoplasty: The nasal bones-anatomy and analysis. Aesthetic Surg. J. 2015, 35, 255–263.
- Kim, D.; Kim, Y.H.; Kwon, S. Enhanced nasal drug delivery efficiency by increasing mechanical loading using hypergravity. Sci. Rep. 2018, 8, 1–9.
- Bitter, C.; Suter-Zimmermann, K.; Surber, C. Nasal drug delivery in humans. Curr. Probl. Dermatol. 2011, 40, 20–35.
- Mujawar, N.; Ghatage, S.; Navale, S.; Sankpal, B.; Patil, S.; Patil, S. Nasal Drug Delivery: Problem Solution and Its Application. J. Curr. Pharma Res. 2014, 4, 1231–1245.
- Gosau, M.; Rink, D.; Driemel, O.; Draenert, F.G. Maxillary sinus anatomy: A cadaveric study with clinical implications. Anat. Rec. 2009, 292, 352–354.
- Inoue, D.; Tanaka, A.; Kimura, S.; Kiriyama, A.; Katsumi, H.; Yamamoto, A.; Ogawara, K.I.; Kimura, T.; Higaki, K.; Yutani, R.; et al. The relationship between in vivo nasal drug clearance and in vitro nasal mucociliary clearance: Application to the prediction of nasal drug absorption. Eur. J. Pharm. Sci. 2018, 117, 21–26.
- Tomazic, P.V.; Darnhofer, B.; Birner-Gruenberger, R. Nasal mucus proteome and its involvement in allergic rhinitis. Expert Rev. Proteom. 2020, 17, 191–199.
- Evans, C.M.; Koo, J.S. Airway mucus: The good, the bad, the sticky. Pharmacol. Ther. 2009, 121, 332–348.
- Roger, D.F. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir. Care 2007, 52, 1134–1146.
- Charlton, S.; Jones, N.S.; Davis, S.S.; Illum, L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur. J. Pharm. Sci. 2007, 30, 295–302.
- Ali, A.; Wahlgren, M.; Rembratt-Svensson, B.; Daftani, A.; Falkman, P.; Wollmer, P.; Engblom, J. Dehydration affects drug transport over nasal mucosa. Drug Deliv. 2019, 26, 831–840.
- Illum, L. Nasal drug delivery: New developments and strategies. Drug Discov. Today 2002, 7, 1184–1189.
- Costa, C.; Moreira, J.N.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J. Control. Release 2019, 295, 187–200.
- Costantino, H.R.; Illum, L.; Brandt, G.; Johnson, P.H.; Quay, S.C. Intranasal delivery: Physicochemical and therapeutic aspects. Int. J. Pharm. 2007, 337, 1–24.
- Tanaka, A.; Furubayashi, T.; Enomura, Y.; Hori, T.; Shimomura, R.; Maeda, C.; Kimura, S.; Inoue, D.; Kusamori, K.; Katsumi, H.; et al. Nasal drug absorption from powder formulations: Effect of fluid volume changes on the mucosal surface. Biol. Pharm. Bull. 2017, 40, 212–219.
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757.
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665.
- Chen, Y.; Cheng, G.; Hu, R.; Chen, S.; Lu, W.; Gao, S.; Xia, H.; Wang, B.; Sun, C.; Nie, X.; et al. A Nasal Temperature and pH Dual-Responsive In Situ Gel Delivery System Based on Microemulsion of Huperzine A: Formulation, Evaluation, and In Vivo Pharmacokinetic Study. AAPS PharmSciTech 2019, 20, 1–12.
- Youssef, N.A.H.A.; Kassem, A.A.; Farid, R.M.; Ismail, F.A.; EL-Massik, M.A.E.; Boraie, N.A. A novel nasal almotriptan loaded solid lipid nanoparticles in mucoadhesive in situ gel formulation for brain targeting: Preparation, characterization and in vivo evaluation. Int. J. Pharm. 2018, 548, 609–624.
- Omar, M.M.; Eleraky, N.E.; El Sisi, A.M.; Ali Hasan, O. Development and Evaluation of in-situ Nasal Gel Formulations of Nanosized Transferosomal Sumatriptan: Design, Optimization, in vitro and in vivo Evaluation. Drug Des. Dev. Ther. 2019, 13, 4413–4430.
- Londhe, V.; Krishnan, S. Formulation, Evaluation, and Pharmacodynamic Investigation of Ziprasidone-b-cyclodextrin In-Situ Nasal Gel. Proceedings 2020, 78, 42.
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A mucoadhesive, thermoreversible in situ nasal gel of geniposide for neurodegenerative diseases. PLoS ONE 2017, 12, e0189478.
- Salatin, S.; Barar, J.; Barzegar-Jalali, M.; Adibkia, K.; Jelvehgari, M. Thermosensitive in situ nanocomposite of rivastigmine hydrogen tartrate as an intranasal delivery system: Development, characterization, ex vivo permeation and cellular studies. Colloids Surf. B Biointerfaces 2017, 159, 629–638.
- Altuntaş, E.; Yener, G. Formulation and Evaluation of Thermoreversible In Situ Nasal Gels Containing Mometasone Furoate for Allergic Rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682.
- Durgapal, S.; Rana, M.; Mukhopadhyay, S.; Rana, A.J.; Goswami, L.; Joshi, S. Formulation and Evaluation of in-Situ Nasal Gel of Montelukast Sodium for the Effective Treatment of Asthma. Int. J. Pharm. Sci. Res. 2018, 9, 2792.
- Khandagale, P.M.; Rokade, M.M.; Phadtare, D.G. Formulation Development and Evaluation of Nasal In- Situ Gel of Hydrocortisone. Asian J. Pharm. Technol. 2018, 8, 92–102.
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35.
- Belgamwar, A.; Khan, S.; Yeole, P. Intranasal chitosan-g-HPβCD nanoparticles of efavirenz for the CNS targeting. Artif. Cells Nanomed. Biotechnol. 2018, 46, 374–386.
- Wilson, B.; Mohamed Alobaid, B.N.; Geetha, K.M.; Jenita, J.L. Chitosan nanoparticles to enhance nasal absorption and brain targeting of sitagliptin to treat Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102176.
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280.
- Ahmed, O.A.A.; Badr-Eldin, S.M. In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: Formulation, characterization, and in vivo performance. Int. J. Nanomed. 2018, 13, 6325–6335.
- Kammoun, A.K.; Khedr, A.; Hegazy, M.A.; Almalki, A.J.; Hosny, K.M.; Abualsunun, W.A.; Murshid, S.S.A.; Bakhaidar, R.B. Formulation, optimization, and nephrotoxicity evaluation of an antifungal in situ nasal gel loaded with voriconazole–clove oil transferosomal nanoparticles. Drug Deliv. 2021, 28, 2229–2240.
- Boche, M.; Pokharkar, V. Quetiapine Nanoemulsion for Intranasal Drug Delivery: Evaluation of Brain-Targeting Efficiency. AAPS PharmSciTech 2017, 18, 686–696.
- Belgamwar, A.V.; Khan, S.A.; Yeole, P.G. Intranasal dolutegravir sodium loaded nanoparticles of hydroxypropyl-beta-cyclodextrin for brain delivery in Neuro-AIDS. J. Drug Deliv. Sci. Technol. 2019, 52, 1008–1020.
- Pashirova, T.N.; Zueva, I.V.; Petrov, K.A.; Lukashenko, S.S.; Nizameev, I.R.; Kulik, N.V.; Voloshina, A.D.; Almasy, L.; Kadirov, M.K.; Masson, P.; et al. Mixed cationic liposomes for brain delivery of drugs by the intranasal route: The acetylcholinesterase reactivator 2-PAM as encapsulated drug model. Colloids Surf. B Biointerfaces 2018, 171, 358–367.
- Tai, J.; Han, M.; Lee, D.; Park, I.-H.; Lee, S.H.; Kim, T.H. Different Methods and Formulations of Drugs and Vaccines for Nasal Administration. Pharmaceutics 2022, 14, 1073.
- Dumkliang, E.; Pamornpathomkul, B.; Patrojanasophon, P.; Ngawhirunpat, T.; Rojanarata, T.; Yoksan, S.; Opanasopit, P. Feasibility of chitosan-based nanoparticles approach for intranasal immunisation of live attenuated Japanese encephalitis vaccine. Int. J. Biol. Macromol. 2021, 183, 1096–1105.
- Oliveira Gomes, D.C.; Lilian da Silva Costa Souza, B.; Schwedersky, R.P.; Covre, L.P.; Leonel de Matos Guedes, H.; Lopes, U.G.; Inês Ré, M.; Rossi-Bergmann, B. Intranasal immunization with chitosan microparticles enhances lack-dna vaccine protection and induces specific long-lasting immunity against visceral leishmaniasis. Microbes Infect. 2021, 24, 104884.
- Sinani, G.; Sessevmez, M.; Gök, M.K.; Özgümüş, S.; Alpar, H.O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 569, 118592.
- Bedford, J.G.; Caminschi, I.; Wakim, L.M. Intranasal delivery of a chitosan-hydrogel vaccine generates nasal tissue resident memory CD8+ T cells that are protective against influenza virus infection. Vaccines 2020, 8, 572.
- Dabaghian, M.; Latifi, A.M.; Tebianian, M.; NajmiNejad, H.; Ebrahimi, S.M. Nasal vaccination with r4M2e.HSP70c antigen encapsulated into N-trimethyl chitosan (TMC) nanoparticulate systems: Preparation and immunogenicity in a mouse model. Vaccine 2018, 36, 2886–2895.
- Pirouzmand, H.; Khameneh, B.; Tafaghodi, M. Immunoadjuvant potential of cross-linked dextran microspheres mixed with chitosan nanospheres encapsulated with tetanus toxoid. Pharm. Biol. 2017, 55, 212–217.
- Kabiri, M.; Bolourian, H.; Dehghan, S.; Tafaghodi, M. The dry powder formulation of mixed cross-linked dextran microspheres and tetanus toxoid-loaded trimethyl chitosan nanospheres as a potent adjuvant for nasal delivery system. Iran. J. Basic Med. Sci. 2020, 24, 116–122.
- Bento, D.; Jesus, S.; Lebre, F.; Gonçalves, T.; Borges, O. Chitosan plus compound 48/80: Formulation and preliminary evaluation as a hepatitis B vaccine adjuvant. Pharmaceutics 2019, 11, 72.
- Najafi, A.; Ghazvini, K.; Sankian, M.; Gholami, L.; Amini, Y.; Zare, S.; Khademi, F.; Tafaghodi, M. T helper type 1 biased immune responses by PPE17 loaded core-shell alginate-chitosan nanoparticles after subcutaneous and intranasal administration. Life Sci. 2021, 282, 119806.
- Mosafer, J.; Sabbaghi, A.H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221.
- Dehghan, S.; Kheiri, M.T.; Abnous, K.; Eskandari, M.; Tafaghodi, M. Preparation, characterization and immunological evaluation of alginate nanoparticles loaded with whole inactivated influenza virus: Dry powder formulation for nasal immunization in rabbits. Microb. Pathog. 2018, 115, 74–85.
- Sinani, G.; Sessevmez, M.; Koray Gök, M.; Özgümüş, S.; Okyar, A.; Oya Alpar, H.; Cevher, E. Nasal vaccination with poly(β-amino ester)-poly(D,L-lactide-co-glycolide) hybrid nanoparticles. Int. J. Pharm. 2017, 529, 1–14.
- Yuki, Y.; Uchida, Y.; Sawada, S.I.; Nakahashi-Ouchida, R.; Sugiura, K.; Mori, H.; Yamanoue, T.; Machita, T.; Honma, A.; Kurokawa, S.; et al. Characterization and Specification of a Trivalent Protein-Based Pneumococcal Vaccine Formulation Using an Adjuvant-Free Nanogel Nasal Delivery System. Mol. Pharm. 2021, 18, 1582–1592.
- Kong, Q.; Kitaoka, M.; Tahara, Y.; Wakabayashi, R.; Kamiya, N.; Goto, M. Solid-in-oil nanodispersions for intranasal vaccination: Enhancement of mucosal and systemic immune responses. Int. J. Pharm. 2019, 572, 118777.
- Bonaccorso, A.; Carbone, C.; Tomasello, B.; Italiani, P.; Musumeci, T.; Puglisi, G.; Pignatello, R. Optimization of dextran sulfate/poly-L-lysine based nanogels polyelectrolyte complex for intranasal ovalbumin delivery. J. Drug Deliv. Sci. Technol. 2021, 65, 102678.
