Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Myeloperoxidase (MPO), also called hydrogen peroxide oxidoreductase with a particular (EC 1.11.1.7), is an enzyme found in the primary granules of granulocytic cells (neutrophils, eosinophils, and, to a lesser extent, monocytes). Lymphocytes lack MPO enzyme activity. However, the most common sources are neutrophils, where the enzyme is located at the lysosomal level in the azurophil granules.
- halogenative stress
- myeloperoxidase MPO
- pathologies
1. Introduction
Myeloperoxidase (MPO) is the major component of white blood cells in humans [1]. These leukocytes recognise microorganisms through various receptors that act by stimulating the migration of the cells to the site of infection, promoting the phagocytosis of the microorganisms and stimulating the production of biocidal substances that destroy the microorganisms. A second microbicidal mechanism used by activated leukocytes occurs during the respiratory burst, which involves reducing molecular oxygen to reactive oxygen intermediates (ROS), such as superoxide radicals •O2−, using the reduced form of NADPH [2]. The superoxide undergoes a disproportionate reaction to give oxygen (O2) and hydrogen peroxide (H2O2), and the latter is used by the MPO enzyme to convert the normally very unreactive halide ions into hypohalous acids that are relatively strong oxidising agents and toxic to bacteria [3]. When intense leukocyte activation occurs, ROS, nitric oxide, and lysosomal enzymes are released, which can injure normal host tissues [4].
MPO is a marker and mediator of inflammation and oxidative stress. An elevated myeloperoxidase level is associated with increased risk, prevalence, and severity, and predicts a poor prognosis in patients with cardiovascular disease. The most common CVD-related actions of MPO are: (i) generation of dysfunctional atherogenic lipoproteins; (ii) reduced NO availability; (iii) endothelial dysfunction; (iv) impaired vasoreactivity; and (v) the instability of the atherosclerotic plaque. This links the MPO levels to the pathophysiology of CVD. Thus, MPO can be considered a mediator or a tool through which inflammation promotes CVD at the molecular and cellular level. MPO can damage host tissue by generating reactive halogenating and nitrating agents [5].
MPO has a detrimental effect during chronic inflammation, and inflammation is reduced in conditions of MPO deficiency. Indeed, this has been observed in many acute and chronic inflammatory diseases. In cases of inflammatory response to non-infectious stimuli (in the absence of pathogens), several recent studies have shown an increase in the inflammatory process associated with the level of MPO.
Neutrophils and macrophages contribute to myocardial and other tissue injury resulting from post-ischemic reperfusion and inflammation [6]. These actions are mainly due to the production by these cells of HOCl/ClO−, superoxide •O2−, and derivatives of •NO [7][8][9][10].
MPO also has other catalytic activities [11]: (a) it reacts with the superoxide •O2− generating the oxo-myeloperoxidase which, in the presence of H2O2, forms “Compound II”; (b) it complexes •NO and catalyses the production of the •NO2 radical [9][10][11], using the nitrite ion as a substrate; (c) it peroxidises different oxidisable organic molecules, forming the corresponding free radicals [12][13][14]; and (d) it hydroxylates aromatic molecules [15][16].
Lipoamide dehydrogenase (LADH) is a mitochondrial enzyme that is very important for energy generation in cardiac muscle. It is inhibited by oxygen free radicals, making it an optimal target for the active species generated by MPO [15][16][17][18]. These species inactivate LADH and thus may contribute to tissue damage by other oxy-radicals (Fenton reaction) as a consequence of post-ischemic reperfusion or inflammation. Compounds with thiol groups, some of which are used therapeutically, counteract the pro-oxidant action of MPO-dependent systems, as shown in Figure 1.
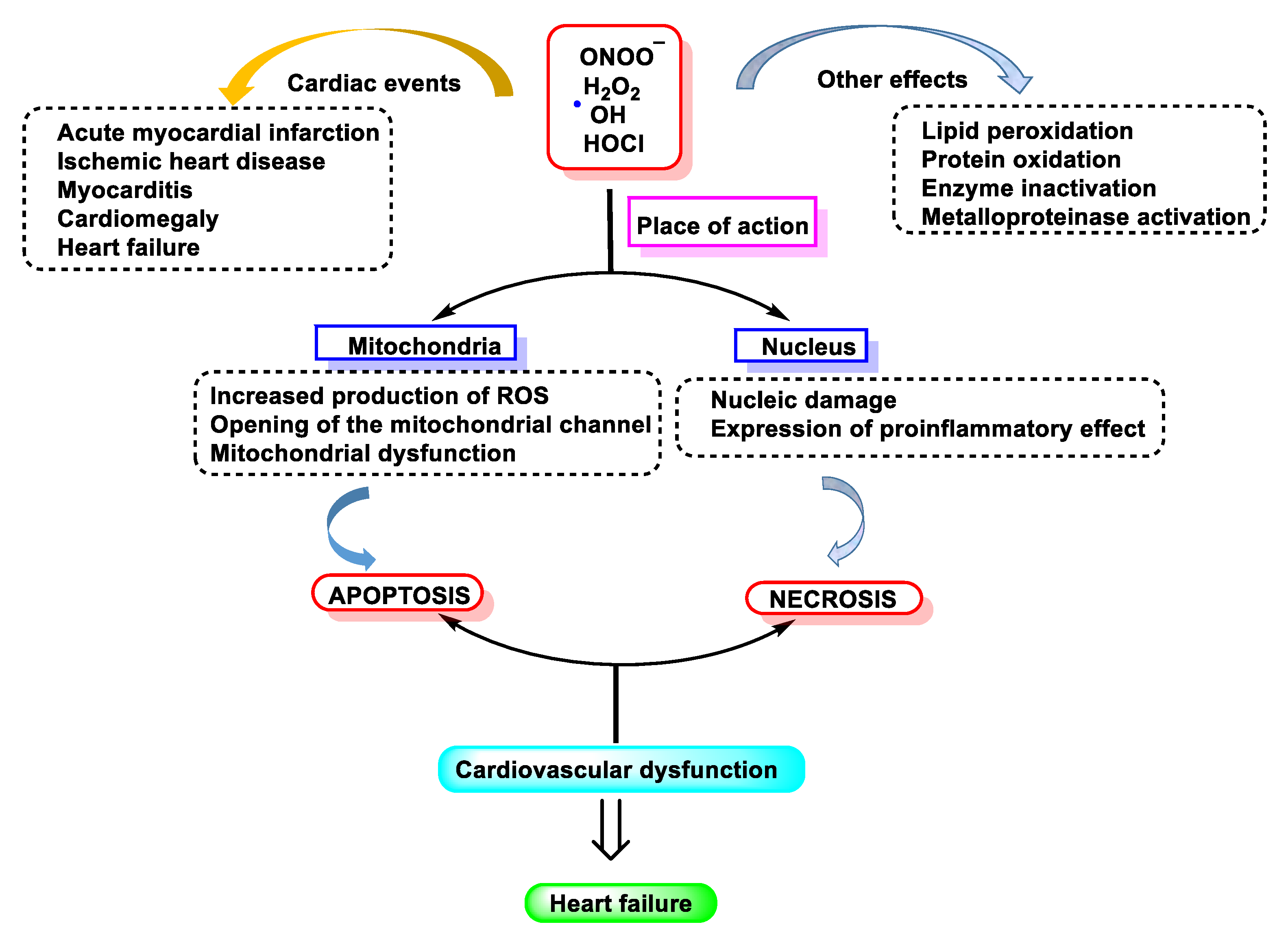
Figure 1. Free radical-induced damage to the cardiovascular system.
2. MPO in Human Diseases
MPO is linked to many aspects of human cardiovascular disease and it is believed that this enzyme acts on both the initiation and propagation of cardiac pathologies [19]. The latter findings agree with results of many other studies that support the concept that MPO plays an important role in the pathogenesis of atherosclerosis and cardiovascular disease. Evidence supports the fact that MPO plays a very important role in the pathogenesis of atherosclerosis because it contributes to vascular dysfunction during acute inflammation by modulating the endothelial NO bioavailability [20]. A recent animal model study of ischaemia-related myocardial damage revealed increases in MPO in arrhythmogenic left ventricular remodelling, as manifested by connexin 43 rupture due to MMP-7 activation and increased post-ischaemic ventricular fibrosis [21]. Thus, MPO contributes to vascular dysfunction by virtue of its capacity to generate potent ROS and to promote the activity of matrix metalloproteinase MMPs [22]
The accumulation of LDL-derived cholesterol in the arterial wall triggers atherosclerosis, the main cause of cardiovascular disease. HDL, on the other hand, delays atherosclerosis by promoting cholesterol efflux. It has been proposed that HDL loses its cardioprotective effects in patients suffering from atherosclerosis [23]. One potential pathway involves oxidative damage by MPO; Baohai Shao et al., 2010, demonstrated that HDL from patients with cardiovascular disease contains high levels of 3-chlorotyrosine and 3-nitrotyrosine, two characteristic products of MPO [24].
MPO and HOCl− modified proteins have been detected in diseased renal tissue. Mollenhauer et al., 2017, observed a significant reduction in renal function loss after the reperfusion of chemically damaged kidneys in MPO-KO mice compared to WT mice, demonstrating a contribution of MPO in the induction of organ damage after renal ischaemia-reperfusion by influencing critical factors such as neutrophil extravasation [21].
Chronic inflammation plays a key role in tumour promotion in lung cancer. Amy L. Rymaszewski et al., 2014, in mouse studies demonstrated that neutrophils are critical mediators of tumour promotion in methylcholanthrene (MCA)-initiated and butylated hydroxytoluene (BHT)-promoted lung carcinogenesis and subsequently, they investigated the role of neutrophil MPO activity in the inflammation-promoted model, observing increased protein levels and MPO activity in the lungs of mice-administered BHT. An MPO inhibitor reduced tumour burden.
Rotem Volkman et al., 2019, reviewed the body of evidence linking neutrophil-derived MPO in the pathogenesis of Alzheimer’s disease (AD), verifying this role in an animal model. They reproduced haematological chimerism in the 5XFAD mouse model of AD with MPO-deficient mice, resulting in 5XFAD with haematological MPO deficiency (5XFAD-MPO KO). Behavioural examinations of 5XFAD-MPO KO mice showed a significantly superior performance in spatial learning and memory, associative learning and anxiety/risk assessment behaviour compared to 5XFAD mice transplanted with WT cells (5XFAD-WT). Immunohistochemical and hippocampal mRNA expression analyses showed significantly reduced levels of inflammatory mediators in 5XFAD-MPO KO mice, with no apparent difference in the number of amyloid-β plaques. In addition, immunoblotting and mRNA analyses showed significantly reduced levels of APOE in 5XFAD-MPO KO mice.
Taken together, their analyses indicate a substantial involvement of neutrophil-derived MPO in the pathogenesis of the 5XFAD model of AD and suggest that MPO is a potential therapeutic target in AD.
In recent years, a significant amount of evidence has implicated a role of MPO in the pathogenesis of atherosclerosis. MPO is an enzymatic source of eicosanoids and bioactive lipids and generates atherogenic forms of low- and high-density lipoproteins. These factors demonstrate that increased systemic levels of MPO and its oxidation products predict increased cardiovascular risk. Consequently, interest has focused on the potential of MPO for the development of new mechanisms to analyse its presence as risk markers, as well as therapies to prevent cardiovascular events. Rachel J. Roth Flach et al., 2019, examined the role of MPO inhibitors in the treatment of heart failure and acute coronary syndrome in humans. The results obtained in their study suggest that MPO inhibition does not alter the atherosclerotic plaque area or leukocyte uptake, but rather alters the inflammatory tone of atherosclerotic lesions; therefore, MPO inhibition has utility in promoting atherosclerotic lesion stabilisation and preventing atherosclerotic plaque rupture [25].
3. MPO and Organ Inflammation
MPO can damage the host tissue by generating reactive halogenating and nitrating agents. Lower levels of 3-chlorotyrosine, 3-bromotyrosine, 3-nitrotyrosine, and protein carbamylation are observed at sites of inflammation in MPO-KO mice compared to WT mice. Therefore, since MPO has a detrimental effect during chronic inflammation, it is to be expected that inflammation is reduced under conditions of MPO deficiency. Indeed, this has been observed in many acute and chronic inflammatory diseases [26].
Recent observations extend this perspective and deeply implicate MPO in the regulation of cellular homeostasis, playing a central role in the initiation and propagation of acute and chronic vascular inflammatory disease. Thus, low levels of HOCl interfere with intracellular signalling, as MPO-dependent lipoprotein oxidation modulates its affinity for macrophages and the vascular wall. Simultaneously, MPO-mediated endothelial NO depletion impairs vasodilation and nitrotyrosine (NO2Tyr) formation in the vascular wall may affect the structure and function of matrix proteins [27].
The development of imaging techniques to accurately identify MPO localisation and molecular targets of HOCl in vivo is an important advance. Until recently, the involvement of MPO in inflammatory disease has been inferred by its presence, together with the detection of HOCl biomarkers, in biological fluids or diseased tissues. These results provide valuable information regarding the cell types responsible for MPO release in vivo, along with new insight into potential therapeutic opportunities [28].
4. Summary Table of the Relationship between MPO and Different Diseases
As follows, Table 1 summarises the relationship between MPO and disease (including recent research findings), citing diseases and references.
Table 1. Occurrence of MPO in various diseases.
| Disease Classification | Disease and References |
|---|---|
| Autoimmune Disease | Inflammatory bowel disease/colitis [29][30][31] Rheumatoid arthritis [32][33] Systemic lupus erythematosus [34][35][36] |
| Neuronal Pathology | Alzheimer’s disease [37][38][39] Multiple sclerosis [40][41][42] Neurodegenerative disease [43][44][45] Parkinson’s disease [46][47] Stroke [48][49][50] |
| Cardiovascular Pathology | Atrial fibrillation [51][52] Cardiovascular disease/atherosclerosis [53][54][55] Hypertension [56][57][58] Myocardial infarction [59][60][61] Vascular dysfunction [62][63][64] Asthma [65][66][67] |
| Pulmonary Pathology | Chronic obstructive pulmonary disease [68][69][70] Cystic fibrosis [71][72][73] |
| Miscellaneous | Ageing [74][75][76] Cancer [77][78][79] Chronic kidney disease [80][81][82] Inflammation [5][28][83] Lipoprotein modification [30][61][62] Metabolic syndrome/obesity [84][85][86] Type 2 diabetes [86][87][88] |
Figure 2, below, summarises what has been explained throughout: chlorinated species have mainly been related to pathologies where the cause is linked to molecular alterations and their effect on inflammatory processes, tissue damage, damage to genetic material, apoptosis, etc.
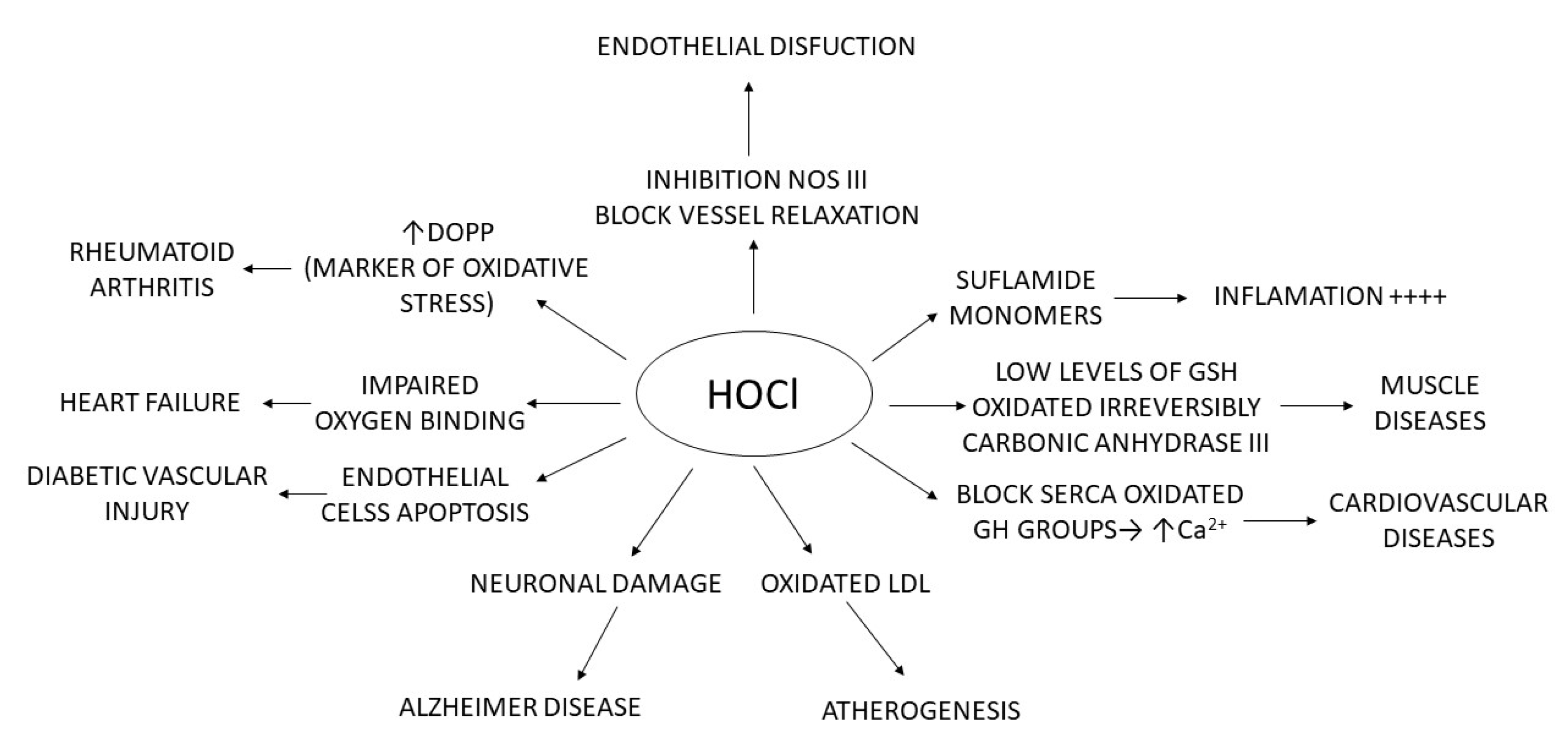
Figure 2. Relationship of HOCl with different pathologies.
5. Myeloperoxidase as a Disease Biomarker
The antibacterial activity of MPO, through the production of HOCl and its controlled release at the site of infection, is of vital importance for its activity to be effective. Its uncontrolled expression overstates inflammation and can lead to tissue damage, even if inflammation does not occur. Several tissue lesions and the pathogenesis of diseases such as rheumatoid arthritis, cardiovascular and liver diseases, diabetes and cancer are linked to MPO-derived HOCl. Therefore, increased MPO activity is a good diagnostic tool for biomarkers of inflammation and oxidative stress [1].
Coordination between several biochemical pathways, including neutrophil activation, •O2− production by NADPH oxidase, and MPO release by exocytosis, leads to clearance of bacterial infection [89], as invading bacteria induce increased H2O2 production by the enzyme superoxide dismutase SOD, whereby MPO produces HOCl. Both products, H2O2 and HOCl, are particularly toxic to invading bacteria. This biochemical phenomenon is called the respiratory burst [90]. When bacterial infection occurs, one of the important mediators of this cascade is formylated peptide, which also acts as a chemoattractant, activating neutrophils via the formylated peptide receptor fPR, a G protein-coupled receptor [91]. The release of H2O2 oxidises various substrates, such as halides (Cl−, Br−), and pseudohalides (thiocyanate SCN—) [3][92]. These oxidant species, under normal physiological circumstances, are toxic to several micro-organisms and play an important role in the immune system. Their excessive or deregulated production of oxidants can cause damage to host cells and lead to various diseases [93][94]. The polycationic character of MPO allows it to bind to negatively charged surfaces of pathogens and causes the destruction of their cell membrane, inducing lysis of the bacteria. This enzyme can also bind to other cell surfaces, such as epithelial cells, macrophages, fibroblasts, endothelial cells, platelets, neutrophils, low-density lipoproteins (LDL), and very low-density lipoproteins (VLDL) [95][96].
6. Measurement of MPO Activity
Currently, there is considerable interest in developing “biomarkers” of MPO activity that can be applied to humans. These involve measuring the end products of oxidative damage in different classes of biomolecules or directly determining the production of HOCl. Different types of equipment are currently available on the market. The most common method of measuring MPO is through enzyme-linked immunosorbent assay ELISA kits. Among the different devices, the following are worth mentioning:
Arigo Biolaboratories Corporation (Hsinchu City, Taiwan), developed a MPO/Myeloperoxidase Activity Assay Kit (Colorimetric) that can be used to measure myeloperoxidase activity in whole neutrophils, neutrophil lysates, tissue homogenates, and plasma (EDTA). This colorimetric device bases its principle of operation on the fact that HOCl rapidly reacts with taurine to produce a stable taurine chloramine product. This step neutralises HOCl, which would otherwise accumulate and inactivate MPO. A stop solution containing catalase is added to stop the catalysis of MPO, removing the hydrogen peroxide. Finally, taurine chloramine reacts with the yellow chromogenic probe TNB, with a decrease in colour indicating increased MPO activity. The concentration of MPO in the samples is then determined by comparing the absorbance of the samples at 405–412 nm with the standard curve.
Abcam, at the Cambridge Biomedical Campus, Cambridge, UK, markets a colorimetric kit with similar characteristics to that described above, notably that it can be used to detect MPO as low as 0.05 mU per well.
Cayman Chemical Company, in Ann Arbor, MI, USA, markets a complete assay for the isolation of neutrophils and the measurement of MPO activity that analyses the release of MPO by activated phagocytes and uses TMB as a chromogenic substrate for MPO, including a specific inhibitor of MPO function to verify specificity. This also includes the reagents necessary to isolate neutrophils from human whole blood.
Celltechgen Laboratory, in Houston, TX, USA, is a state-of-the-art medical testing laboratory service that provides a complete range of tests for the diagnosis, screening, or evaluation of diseases and health conditions and markets an MPO Peroxidation Activity Assay Kit, suitable for use as a high-throughput MPO activity assay. The assay kit oxidises a substrate to generate fluorescence (Ex/Em = 571/585 nm), directly proportional to total peroxidase activity in the sample. The assay is high-throughput adaptable and can detect less than 2 µU of MPO activity. This kit can be used to detect MPO activity as low as 0.5 µU per well.
7. Inhibitors of MPO
The microbicidal activity of the MPO enzyme is due to its ability to oxidise halide or pseudohalide ions (X = Cl−, Br−, I− and SCN−) producing the respective HOX acids. During the phagocytosis of pathogens, MPO is released by azurophilic granules into phagolysosomes but can also be discharged outside phagocytes. Tissue damage that occurs during inflammation is largely due to MPO-derived oxidants. As referenced in previous chapters, this enzyme is a key factor in several conditions, including cardiovascular, inflammatory, neurodegenerative, renal, immune-mediated, and neurodegenerative diseases. Therefore, MPO is an attractive target for therapeutic intervention in the prophylaxis of the aforementioned disorders. The only negative effect that can be expected from MPO inhibitors is a decrease in neutrophil activity against pathogens. MPO is an immunological enzyme that acts in neutrophils. However, MPO is located in azurophil granules, which protect this enzyme from changes in the extracellular environment. Therefore, it is believed that the effect of inhibitors on MPO activity can be easily attenuated by exclusively focusing on extracellular enzymes that are not critical for pathogen eradication but rather are involved in host damage.
Relatively polar MPO inhibitors cannot penetrate neutrophils and are thought to inhibit extracellular enzymes exclusively. The structure and reaction mechanism of MPO is known, allowing a rational strategy for the development of specific inhibitors, with the intention of preserving its activity against bacteria, but hindering its pathophysiological persistent activation during the course of the diseases [97].
There are three approaches to discover and develop such inhibitors:
The first approach is to prepare tiny compounds with a reduction potential of 0.97 V E°′ (A-/AH) 1.35 V. MPO compound I can easily oxidise these molecules, leading to the inactive state MPO compound II. At the same time, they cannot degrade the MPO compound II and cause this inactive form of MPO to build up. The main problem with these inhibitors is that when they are used in living organisms, several biomolecules can act as substrates for MPO and degrade the MPO compound II to restore the original enzyme. As a result, many inhibitors lose their efficacy in vivo.
The second approach focuses on tiny molecules that bind with high affinity to the active site of MPO. These chemicals are designed to strongly interact with the active site residues of the enzyme. The key amino acid that forms a salt bridge or hydrogen bond with the inhibitor is Glu102. In addition, to convert the MPO compound I into the MPO compound II, the inhibitor must have a reduction potential E°′ (A-/AH) of less than 1.35 V. When this type of molecule induces the inactive state of the enzyme, it maintains its contacts with the active site of MPO and prevents additional substrates from entering the active site, leading to the accumulation of MPO compound II by competitive inhibition. Several effective MPO inhibitors, such as aminoalkyl-indole compounds and aryl hydroxamic acid derivatives [98][99][100][101], were prepared using this strategy.
The third approach is to prepare tiny molecules that form covalent bonds after oxidation by MPO and have a relatively high affinity for the enzyme. These compounds are irreversible MPO inhibitors that act by degrading the heme group. Inhibitors that rely on overriding this mechanism are irreversible and form a strong covalent bond with the Fe of the haem centre, blocking the access of H2O2 to the active site, and thus inactivating the enzyme. The second mechanism is based on inducing competition between the inhibitor compound and the enzyme substrate. In this case, the inhibitor either forms a complex with MPO preventing further cycling or acts as a substrate for MPO by forming and accumulating compound II. An alternative approach based on the design of HOCl scavenging compounds can be considered in order to avoid the induced oxidative damage. However, this mechanism will not prevent the peroxidation cycle and the formation of superoxide and hydroxyl radical, which are involved in oxidative stress and tissue injury. Due to the complexity of the MPO catalytic mechanism, the search for an effective inhibitor is still under development [102].
Lifestyle factors are important drivers of chronic diseases such as cardiovascular syndrome, with inflammation being a key factor. MPO is an inflammatory enzyme associated with obesity, hypertension, and heart failure, so its attenuation could have protective effects on multiple organs. Arnold Piek et al., 2019, tested the effects of a novel oral MPO inhibitor AZM198 in an obese/hypertensive mouse model with a cardiac phenotype Treated animals showed therapeutic AZM198 levels of 2.1 µM, corresponding to a 95% inhibition of MPO. AZM198 reduced elevated circulating MPO levels in HFD/AngII mice to normal values. Independently of food intake, body weight gain and fat accumulation were attenuated, along with reduction in visceral adipose tissue (VAT) inflammation and the attenuation of the severity of non-alcoholic steatohepatitis [103].
Most peroxidase enzymes are inhibited by benzoic acid hydrazide (BAH)-containing compounds, but the inhibition mechanism by BAH compounds is unknown. Jiansheng Huang et al., 2015, reported that the MPO inhibition by BAH and 4-(trifluoromethyl)-BAH due to hydrolysis of the ester bond between the MPO heavy chain glutamate 242 ((HC)Glu(242)) residue and the heme pyrrole A ring. In their manuscript, they provide evidence that the destruction of the heme ring does not occur by heme prosthetic group tracking and provides indications that the mechanism of hydrolysis follows a potential attack of the carbonyl of (HC)Glu(242), leading to a rearrangement that causes the release of the vinyl-sulphonium bond between (HC)Met(243) and the pyrrole A-ring [104].
Clinical studies have been only conducted on AZD5904, AZD3241, AZD4831, and PF06282999. The first three chemicals are thioxanthine derivatives, while the fourth is a thiopyrimidinone. The common mechanism of action of these drugs is a thioether bridge between the heme group of the enzyme and the oxidised thioxantine or thiopyrimidinone. Thioxanthins are compounds known as MPO inhibitors and derivatives with the 2-thioxanthin group show the highest activity. Thioxanthine is oxidised by compound I, forming a highly reactive free radical. This radical readily transfers electrons to the heme group of compound II and forms a covalent bond across the sulphur atom to one of the heme’s pyrrole rings [105].
Several naturally occurring compounds possess inhibitory activities against MPO, including polyphenols, melatonin, and flavonoids. Seeds and aerial parts of Peganum harmala L. are widely used in Algeria as anti-inflammatory remedies. Sihem Bensalem et al. evaluated in their study the alkaloids and pure β-carboline compounds present, as well as their possible anti-inflammatory and MPO inhibitory action, concluding that the total alkaloids from seeds and aerial parts strongly inhibited MPO at 20 µg/mL (97 ± 5% and 43 ± 4%, respectively) while, at the same concentration, those from the roots showed very low inhibition (15 ± 6%) [106].
On the other hand, ceruloplasmin is a plasma protein produced by activated hepatocytes and macrophages and is involved in the physiological clearance and inactivation of MPO [107]. In addition to these, some naturally occurring compounds, such as polyphenols, with pronounced antioxidant and anti-inflammatory characteristics, have inhibitory activities against MPO. These compounds include ferulic acid, caffeic acid, resveratrol, chalcones, and gallic acid. [108][109]. Yuko Shiba et al., 2008, examined the MPO inhibitory effects of dietary flavonoids, using a combination of biological assays and theoretical computational studies. Quercetin and the plasma metabolites inhibited the formation of dityrosine catalysed by the MPO enzyme and HL-60 cells in a dose-dependent manner [110].
This entry is adapted from the peer-reviewed paper 10.3390/ijms231810735
References
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Med. Sci. 2018, 6, 33.
- Hurst, J.K. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012, 53, 508–520.
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2010, 48, 8–19.
- Shankar, S.; Mahadevan, A.; Satishchandra, P.; Uday Kumar, R.; Yasha, T.; Santosh, V.; Chandramuki, A.; Ravi, V.; Nath, A. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J. Med. Res. 2005, 121, 468–488.
- Ndrepepa, G. Myeloperoxidase–A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51.
- Kuzuya, T.; Fuji, H.; Hoshida, S.; Nishida, M.; Goshima, K.; Hori, M.; Kamada, T.; Tada, M. Polymorphonuclear leukocytes-induced injury in hypoxic cardiac myocytes. Free Radic. Biol. Med. 1994, 17, 501–510.
- Leone, A.; Palmer, R.; Knowles, R.; Francis, P.; Ashton, D.; Moncada, S. Constitutive and inducible nitric oxide synthases incorporate molecular oxygen into both nitric oxide and citrulline. J. Biol. Chem. 1991, 266, 23790–23795.
- Cooper, C.E.; Odell, E. Interaction of human myeloperoxidase with nitrite. FEBS Lett. 1992, 314, 58–60.
- Klebanoff, S.J. Reactive nitrogen intermediates and antimicrobial activity: Role of nitrite. Free Radic. Biol. Med. 1993, 14, 351–360.
- Van Der Vliet, A.; Eiserich, J.P.; Halliwell, B.; Cross, C.E. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite: A potential additional mechanism of nitric oxide-dependent toxicity. J. Biol. Chem. 1997, 272, 7617–7625.
- Kettle, A.; Winterbourn, C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15.
- O’Brien, P.J. Radical formation during the peroxidase catalyzed metabolism of carcinogens and xenobiotics: The reactivity of these radicals with GSH, DNA, and unsaturated lipid. Free Radic. Biol. Med. 1988, 4, 169–183.
- Eastmond, D.; Smith, M.; Ruzo, L.; Ross, D. Metabolic activation of phenol by human myeloperoxidase and horseradish peroxidase. Mol. Pharmacol. 1986, 30, 674–679.
- Frimat, B.; Gressier, B.; Odou, P.; Brunet, C.; Dine, T.; Luycky, M.; Cazin, M.; Cazin, J. Metabolism of clozapine by human neutrophils: Evidence for a specific oxidation of clozapine by the myeloperoxidase system with inhibition of enzymatic chlorination cycle. Fundam. Clin. Pharmacol. 1997, 11, 267–274.
- Gutierrez-Correa, J.; Stoppani, A. Inactivation of heart dihydrolipoamide dehydrogenase by copper Fenton systems. Effect of thiol compounds and metal chelators. Free Radic. Res. 1995, 22, 239–250.
- Correa, J.G.; Stoppani, A. Catecholamines enhance dihydrolipoamide dehydrogenase inactivation by the copper Fenton system. Enzyme protection by copper chelators. Free Radic. Res. 1996, 24, 311–322.
- Correa, J.G.; Stoppani, A. Inactivation of lipoamide dehydrogenase by cobalt (II) and iron (II) Fenton systems: Effect of metal chelators, thiol compounds and adenine nucleotides. Free Radic. Res. Commun. 1993, 19, 303–314.
- Gutierrez Correa, J.; Biscardi, A.M.; Stoppani, A.O. Inactivación de la lipoamida deshidrogenasa de miocardio por catecolaminas: Protección por captopril y otros tioles. Med. B. Aires 1995, 55, 397–407.
- Anatoliotakis, N.; Deftereos, S.; Bouras, G.; Giannopoulos, G.; Tsounis, D.; Angelidis, C.; Kaoukis, A.; Stefanadis, C. Myeloperoxidase: Expressing inflammation and oxidative stress in cardiovascular disease. Curr. Top. Med. Chem. 2013, 13, 115–138.
- Eiserich, J.P.; Baldus, S.; Brennan, M.-L.; Ma, W.; Zhang, C.; Tousson, A.; Castro, L.; Lusis, A.J.; Nauseef, W.M.; White, C.R. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 2002, 296, 2391–2394.
- Mollenhauer, M.; Friedrichs, K.; Lange, M.; Gesenberg, J.; Remane, L.; Kerkenpaß, C.; Krause, J.; Schneider, J.; Ravekes, T.; Maass, M. Myeloperoxidase mediates postischemic arrhythmogenic ventricular remodeling. Circ. Res. 2017, 121, 56–70.
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67.
- Carr, A.C.; McCall, M.R.; Frei, B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1716–1723.
- Shao, B.; Oda, M.N.; Oram, J.F.; Heinecke, J.W. Myeloperoxidase: An oxidative pathway for generating dysfunctional high-density lipoprotein. Chem. Res. Toxicol. 2010, 23, 447–454.
- Roth Flach, R.J.; Su, C.; Bollinger, E.; Cortes, C.; Robertson, A.W.; Opsahl, A.C.; Coskran, T.M.; Maresca, K.P.; Keliher, E.J.; Yates, P.D. Myeloperoxidase inhibition in mice alters atherosclerotic lesion composition. PLoS ONE 2019, 14, e0214150.
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184.
- Pham, P.; Pham, P.; Pham, P. Patients with diabetes mellitus type 2 and hypomagnesemia may have enhanced glomerular filtration via hypocalcemia. Clin. Nephrol. 2012, 78, 442–448.
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981.
- Chami, B.; Martin, N.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71.
- Garrity-Park, M.; Loftus, E.V., Jr.; Sandborn, W.J.; Smyrk, T.C. Myeloperoxidase immunohistochemistry as a measure of disease activity in ulcerative colitis: Association with ulcerative colitis-colorectal cancer, tumor necrosis factor polymorphism and RUNX3 methylation. Inflamm. Bowel Dis. 2012, 18, 275–283.
- Chami, B.; Ahmad, G.; Schroder, A.; San Gabriel, P.; Witting, P. The role of myeloperoxidase and neutrophil extracellular traps in the pathogenesis of inflammatory bowel disease. Gastroenterology 2021, 160, S5–S6.
- Wang, W.; Jian, Z.; Guo, J.; Ning, X. Increased levels of serum myeloperoxidase in patients with active rheumatoid arthritis. Life Sci. 2014, 117, 19–23.
- Fernandes, R.M.S.N.; Silva, N.P.d.; Sato, E.I. Increased myeloperoxidase plasma levels in rheumatoid arthritis. Rheumatol. Int. 2012, 32, 1605–1609.
- Telles, R.W.; Ferreira, G.A.; Silva, N.P.d.; Sato, E.I. Increased plasma myeloperoxidase levels in systemic lupus erythematosus. Rheumatol. Int. 2010, 30, 779–784.
- Olson, S.; Lee, J.; Poirier, M.; Little, D.; Prince, L.; Baker, T.; Edison, J.; Abbott, K. Anti-myeloperoxidase antibodies associate with future proliferative lupus nephritis. Autoimmune Dis. 2017, 2017, 1872846.
- Carrillo-Vázquez, D.A.; Jardón-Valadez, E.; Torres-Ruiz, J.; Juárez-Vega, G.; Maravillas-Montero, J.L.; Meza-Sánchez, D.E.; Domínguez-López, M.L.; Varela, J.C.A.; Gómez-Martín, D. Conformational changes in myeloperoxidase induced by ubiquitin and NETs containing free ISG15 from systemic lupus erythematosus patients promote a pro-inflammatory cytokine response in CD4+ T cells. J. Transl. Med. 2020, 18, 429.
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J. Neurochem. 2004, 90, 724–733.
- Tzikas, S.; Schlak, D.; Sopova, K.; Gatsiou, A.; Stakos, D.; Stamatelopoulos, K.; Stellos, K.; Laske, C. Increased myeloperoxidase plasma levels in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 39, 557–564.
- Volkman, R.; Ben-Zur, T.; Kahana, A.; Garty, B.Z.; Offen, D. Myeloperoxidase deficiency inhibits cognitive decline in the 5XFAD mouse model of Alzheimer’s disease. Front. Neurosci. 2019, 13, 990.
- Pulli, B.; Bure, L.; Wojtkiewicz, G.R.; Iwamoto, Y.; Ali, M.; Li, D.; Schob, S.; Hsieh, K.L.-C.; Jacobs, A.H.; Chen, J.W. Multiple sclerosis: Myeloperoxidase immunoradiology improves detection of acute and chronic disease in experimental model. Radiology 2015, 275, 480.
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008, 444, 195–198.
- Nagra, R.M.; Becher, B.; Tourtellotte, W.W.; Antel, J.P.; Gold, D.; Paladino, T.; Smith, R.A.; Nelson, J.R.; Reynolds, W.F. Immunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J. Neuroimmunol. 1997, 78, 97–107.
- Pravalika, K.; Sarmah, D.; Kaur, H.; Wanve, M.; Saraf, J.; Kalia, K.; Borah, A.; Yavagal, D.R.; Dave, K.R.; Bhattacharya, P. Myeloperoxidase and neurological disorder: A crosstalk. ACS Chem. Neurosci. 2018, 9, 421–430.
- Ray, R.; Katyal, A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci. Biobehav. Rev. 2016, 68, 611–620.
- Gellhaar, S.; Sunnemark, D.; Eriksson, H.; Olson, L.; Galter, D. Myeloperoxidase-immunoreactive cells are significantly increased in brain areas affected by neurodegeneration in Parkinson’s and Alzheimer’s disease. Cell Tissue Res. 2017, 369, 445–454.
- Choi, D.-K.; Pennathur, S.; Perier, C.; Tieu, K.; Teismann, P.; Wu, D.-C.; Jackson-Lewis, V.; Vila, M.; Vonsattel, J.-P.; Heinecke, J.W. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J. Neurosci. 2005, 25, 6594–6600.
- Jucaite, A.; Svenningsson, P.; Rinne, J.O.; Cselenyi, Z.; Varnäs, K.; Johnström, P.; Amini, N.; Kirjavainen, A.; Helin, S.; Minkwitz, M. Effect of the myeloperoxidase inhibitor AZD3241 on microglia: A PET study in Parkinson’s disease. Brain 2015, 138, 2687–2700.
- Cojocaru, I.M.; Cojocaru, M.; Iliescu, I.; Botnaru, L.; Gurban, C.V.; Sfrijan, F.; Tanasescu, R. Plasma myeloperoxidase levels in patients with acute ischemic stroke. Rom. J. Intern. Med. 2010, 48, 101–104.
- Tay, A.; Tamam, Y.; Yokus, B.; Ustundag, M.; Orak, M. Serum myeloperoxidase levels in predicting the severity of stroke and mortality in acute ischemic stroke patients. Eur. Rev. Med. Pharm. Sci. 2015, 19, 1983–1988.
- Yu, G.; Liang, Y.; Huang, Z.; Jones, D.W.; Pritchard, K.A.; Zhang, H. Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J. Neuroinflamm. 2016, 13, 119.
- Li, S.-B.; Yang, F.; Jing, L.; Ma, J.; Jia, Y.-D.; Dong, S.-Y.; Zheng, W.-F.; Zhao, L.-S. Myeloperoxidase and risk of recurrence of atrial fibrillation after catheter ablation. J. Investig. Med. 2013, 61, 722–727.
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474.
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase, modified lipoproteins, and atherogenesis. J. Lipid Res. 2009, 50, S346–S351.
- Tsimikas, S. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am. J. Cardiol. 2006, 98, S9–S17.
- Zhang, R.; Brennan, M.-L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association between myeloperoxidase levels and risk of coronary artery disease. JAMA 2001, 286, 2136–2142.
- Klinke, A.; Berghausen, E.; Friedrichs, K.; Molz, S.; Lau, D.; Remane, L.; Berlin, M.; Kaltwasser, C.; Adam, M.; Mehrkens, D. Myeloperoxidase aggravates pulmonary arterial hypertension by activation of vascular Rho-kinase. JCI Insight 2018, 3, e97530.
- Van der Zwan, L.P.; Scheffer, P.G.; Dekker, J.M.; Stehouwer, C.D.; Heine, R.J.; Teerlink, T. Hyperglycemia and oxidative stress strengthen the association between myeloperoxidase and blood pressure. Hypertension 2010, 55, 1366–1372.
- Rocha-Penha, L.; Caldeira-Dias, M.; Tanus-Santos, J.E.; de Carvalho Cavalli, R.; Sandrim, V.C. Myeloperoxidase in hypertensive disorders of pregnancy and its relation with nitric oxide. Hypertension 2017, 69, 1173–1180.
- Mocatta, T.J.; Pilbrow, A.P.; Cameron, V.A.; Senthilmohan, R.; Frampton, C.M.; Richards, A.M.; Winterbourn, C.C. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 1993–2000.
- Rees, M.D.; Pattison, D.I.; Davies, M.J. Oxidation of heparan sulphate by hypochlorite: Role of N-chloro derivatives and dichloramine-dependent fragmentation. Biochem. J. 2005, 391, 125–134.
- Omran, M.M.; Zahran, F.M.; Kadry, M.; Belal, A.A.; Emran, T.M. Role of myeloperoxidase in early diagnosis of acute myocardial infarction in patients admitted with chest pain. J. Immunoass. Immunochem. 2018, 39, 337–347.
- Abdo, A.I.; Rayner, B.S.; van Reyk, D.M.; Hawkins, C.L. Low-density lipoprotein modified by myeloperoxidase oxidants induces endothelial dysfunction. Redox Biol. 2017, 13, 623–632.
- Manchanda, K.; Kolarova, H.; Kerkenpaß, C.; Mollenhauer, M.; Vitecek, J.; Rudolph, V.; Kubala, L.; Baldus, S.; Adam, M.; Klinke, A. MPO (myeloperoxidase) reduces endothelial glycocalyx thickness dependent on its cationic charge. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1859–1867.
- Vita, J.A.; Brennan, M.-L.; Gokce, N.; Mann, S.A.; Goormastic, M.; Shishehbor, M.H.; Penn, M.S.; Keaney, J.F., Jr.; Hazen, S.L. Serum myeloperoxidase levels independently predict endothelial dysfunction in humans. Circulation 2004, 110, 1134–1139.
- Caramori, G.; Papi, A. Oxidants and asthma. Thorax 2004, 59, 170–173.
- Ekmekci, O.; Donma, O.; Sardoğan, E.; Yildirim, N.; Uysal, O.; Demirel, H.; Demir, T. Iron, nitric oxide, and myeloperoxidase in asthmatic patients. Biochemistry 2004, 69, 462–467.
- Jatakanon, A.; Uasuf, C.; Maziak, W.; Lim, S.; Chung, K.F.; Barnes, P.J. Neutrophilic inflammation in severe persistent asthma. Am. J. Respir. Crit. Care Med. 1999, 160, 1532–1539.
- O’Donnell, C.; Newbold, P.; White, P.; Thong, B.; Stone, H.; Stockley, R.A. 3-Chlorotyrosine in sputum of COPD patients: Relationship with airway inflammation. COPD J. Chronic Obstr. Pulm. Dis. 2010, 7, 411–417.
- Zhu, A.; Ge, D.; Zhang, J.; Teng, Y.; Yuan, C.; Huang, M.; Adcock, I.M.; Barnes, P.J.; Yao, X. Sputum myeloperoxidase in chronic obstructive pulmonary disease. Eur. J. Med. Res. 2014, 19, 12.
- Andelid, K.; Glader, P.; Jirholt, P.; Gjertsson, I.; Jansson, A.E.; Lindén, A. Systemic myeloperoxidase in COPD. Respir. Med. 2013, 107, S4.
- Kettle, A.J.; Chan, T.; Osberg, I.; Senthilmohan, R.; Chapman, A.L.; Mocatta, T.J.; Wagener, J.S. Myeloperoxidase and protein oxidation in the airways of young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 2004, 170, 1317–1323.
- Van der Vliet, A.; Nguyen, M.N.; Shigenaga, M.K.; Eiserich, J.P.; Marelich, G.P.; Cross, C.E. Myeloperoxidase and protein oxidation in cystic fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L537–L546.
- Vasu, V.T.; De Cruz, S.J.; Houghton, J.S.; Hayakawa, K.A.; Morrissey, B.M.; Cross, C.E.; Eiserich, J.P. Evaluation of thiol-based antioxidant therapeutics in cystic fibrosis sputum: Focus on myeloperoxidase. Free Radic. Res. 2011, 45, 165–176.
- Casciaro, M.; Di Salvo, E.; Pace, E.; Ventura-Spagnolo, E.; Navarra, M.; Gangemi, S. Chlorinative stress in age-related diseases: A literature review. Immun. Ageing 2017, 14, 21.
- Alikhan, M.A.; Jaw, J.; Shochet, L.R.; Robson, K.J.; Ooi, J.D.; Brouwer, E.; Heeringa, P.; Holdsworth, S.R.; Kitching, A.R. Ageing enhances cellular immunity to myeloperoxidase and experimental anti-myeloperoxidase glomerulonephritis. Rheumatology 2022, 61, 2132–2143.
- Lu, H.; Xu, S.; Liang, X.; Dai, Y.; Huang, Z.; Ren, Y.; Lin, J.; Liu, X. Advanced glycated end products alter neutrophil effect on regulation of CD4+ T cell differentiation through induction of myeloperoxidase and neutrophil elastase activities. Inflammation 2019, 42, 559–571.
- Fedeles, B.I.; Freudenthal, B.D.; Yau, E.; Singh, V.; Chang, S.-c.; Li, D.; Delaney, J.C.; Wilson, S.H.; Essigmann, J.M. Intrinsic mutagenic properties of 5-chlorocytosine: A mechanistic connection between chronic inflammation and cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4571–E4580.
- Rymaszewski, A.L.; Tate, E.; Yimbesalu, J.P.; Gelman, A.E.; Jarzembowski, J.A.; Zhang, H.; Pritchard, K.A., Jr.; Vikis, H.G. The role of neutrophil myeloperoxidase in models of lung tumor development. Cancers 2014, 6, 1111–1127.
- Weitzman, S.A.; Gordon, L.I. Inflammation and cancer: Role of phagocyte-generated oxidants in carcinogenesis. Blood 1990, 76, 655–663.
- Kisic, B.; Miric, D.; Dragojevic, I.; Rasic, J.; Popovic, L. Role of myeloperoxidase in patients with chronic kidney disease. Oxidative Med. Cell. Longev. 2016, 2016, 1069743.
- Lehners, A.; Lange, S.; Niemann, G.; Rosendahl, A.; Meyer-Schwesinger, C.; Oh, J.; Stahl, R.; Ehmke, H.; Benndorf, R.; Klinke, A. Myeloperoxidase deficiency ameliorates progression of chronic kidney disease in mice. Am. J. Physiol. Ren. Physiol. 2014, 307, F407–F417.
- Madhusudhana Rao, A.; Anand, U.; Anand, C. Myeloperoxidase in chronic kidney disease. Indian J. Clin. Biochem. 2011, 26, 28–31.
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting myeloperoxidase (MPO) mediated oxidative stress and inflammation for reducing brain ischemia injury: Potential application of natural compounds. Front. Physiol. 2020, 11, 433.
- Wang, Q.; Xie, Z.; Zhang, W.; Zhou, J.; Wu, Y.; Zhang, M.; Zhu, H.; Zou, M.-H. Myeloperoxidase deletion prevents high-fat diet–induced obesity and insulin resistance. Diabetes 2014, 63, 4172–4185.
- Zaki, M.; Basha, W.; Reyad, H.; Mohamed, R.; Hassan, N.; Kholousi, S. Association between myeloperoxidase levels and risk of insulin resistance in Egyptian obese women. Open Access Maced. J. Med. Sci. 2018, 6, 629.
- Qaddoumi, M.G.; Alanbaei, M.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Channanath, A.; Thanaraj, T.A.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes. Sci. Rep. 2020, 10, 6170.
- García, A.G.; Rodríguez, M.R.; Alonso, C.G.; Ochoa, D.Y.R.; Aguilar, C.A. Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab. J. 2015, 39, 59–65.
- Heinecke, J.W.; Goldberg, I.J. Myeloperoxidase: A therapeutic target for preventing insulin resistance and the metabolic sequelae of obesity? Diabetes 2014, 63, 4001–4003.
- Nauseef, W.M. Myeloperoxidase in human neutrophil host defence. Cell. Microbiol. 2014, 16, 1146–1155.
- El Kebir, D.; József, L.; Pan, W.; Filep, J.n.G. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circ. Res. 2008, 103, 352–359.
- Dorward, D.A.; Lucas, C.D.; Chapman, G.B.; Haslett, C.; Dhaliwal, K.; Rossi, A.G. The role of formylated peptides and formyl peptide receptor 1 in governing neutrophil function during acute inflammation. Am. J. Pathol. 2015, 185, 1172–1184.
- Futosi, K.; Fodor, S.; Mócsai, A. Reprint of Neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 1185–1197.
- Hawkins, C.L.; Pattison, D.I.; Stanley, N.R.; Davies, M.J. Tryptophan residues are targets in hypothiocyanous acid-mediated protein oxidation. Biochem. J. 2008, 416, 441–452.
- Britigan, B.E.; Ratcliffe, H.R.; Buettner, G.R.; Rosen, G.M. Binding of myeloperoxidase to bacteria: Effect on hydroxyl radical formation and susceptibility to oxidant-mediated killing. Biochim. Biophys. Acta BBA Gen. Subj. 1996, 1290, 231–240.
- Haegens, A.; Vernooy, J.H.; Heeringa, P.; Mossman, B.T.; Wouters, E.F. Myeloperoxidase modulates lung epithelial responses to pro-inflammatory agents. Eur. Respir. J. 2008, 31, 252–260.
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.-J.; Schröder, C.; Benten, D.; Lau, D. Myeloperoxidase attracts neutrophils by physical forces. Blood J. Am. Soc. Hematol. 2011, 117, 1350–1358.
- Lazarevic-Pasti, T.; Leskovac, A.; Vasic, V. Myeloperoxidase inhibitors as potential drugs. Curr. Drug Metab. 2015, 16, 168–190.
- Panasenko, O.; Torkhovskaya, T.; Gorudko, I.; Sokolov, A. The role of halogenative stress in atherogenic modification of low-density lipoproteins. Biochemistry 2020, 85, 34–55.
- Spickett, C.M.; Jerlich, A.; Panasenko, O.M.; Arnhold, J.; Pitt, A.R.; Stelmaszyńska, T.; Schaur, R.J. The reactions of hypochlorous acid, the reactive oxygen species produced by myeloperoxidase, with lipids. Acta Biochim. Pol. 2000, 47, 889–899.
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 1442–1452.
- Yang, P.; Subbaiah, P.V. Regulation of hepatic lipase activity by sphingomyelin in plasma lipoproteins. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2015, 1851, 1327–1336.
- Galijasevic, S. The development of myeloperoxidase inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 1–7.
- Piek, A.; Koonen, D.P.; Schouten, E.-M.; Lindtstedt, E.L.; Michaëlsson, E.; de Boer, R.A.; Silljé, H.H. Pharmacological myeloperoxidase (MPO) inhibition in an obese/hypertensive mouse model attenuates obesity and liver damage, but not cardiac remodeling. Sci. Rep. 2019, 9, 18765.
- Huang, J.; Smith, F.; Panizzi, J.R.; Goodwin, D.C.; Panizzi, P. Inactivation of myeloperoxidase by benzoic acid hydrazide. Arch. Biochem. Biophys. 2015, 570, 14–22.
- Furtmüller, P.G.; Arnhold, J.; Jantschko, W.; Pichler, H.; Obinger, C. Redox properties of the couples compound I/compound II and compound II/native enzyme of human myeloperoxidase. Biochem. Biophys. Res. Commun. 2003, 301, 551–557.
- Bensalem, S.; Soubhye, J.; Aldib, I.; Bournine, L.; Nguyen, A.T.; Vanhaeverbeek, M.; Rousseau, A.; Boudjeltia, K.Z.; Sarakbi, A.; Kauffmann, J.M. Inhibition of myeloperoxidase activity by the alkaloids of Peganum harmala L.(Zygophyllaceae). J. Ethnopharmacol. 2014, 154, 361–369.
- Segelmark, M.; Persson, B.; Hellmark, T.; Wieslander, J. Binding and inhibition of myeloperoxidase (MPO): A major function of ceruloplasmin? Clin. Exp. Immunol. 1997, 108, 167–174.
- Kohnen, S.; Franck, T.; Van Antwerpen, P.; Zouaoui Boudjeltia, K.; Mouithys-Mickalad, A.; Deby, C.; Moguilevsky, N.; Deby-Dupont, G.; Lamy, M.; Serteyn, D. Resveratrol inhibits the activity of equine neutrophil myeloperoxidase by a direct interaction with the enzyme. J. Agric. Food Chem. 2007, 55, 8080–8087.
- Zeraik, M.L.; Ximenes, V.F.; Regasini, L.O.; Dutra, L.; Silva, D.H.S.; Fonseca, L.; Coelho, D.; Machado, S.; Bolzani, V.d.S. 4′-Aminochalcones as novel inhibitors of the chlorinating activity of myeloperoxidase. Curr. Med. Chem. 2012, 19, 5405–5413.
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Naito, M.; Kawabata, K.; Ishisaka, A.; Terao, J. Flavonoids as substrates and inhibitors of myeloperoxidase: Molecular actions of aglycone and metabolites. Chem. Res. Toxicol. 2008, 21, 1600–1609.
This entry is offline, you can click here to edit this entry!
