Cancer is one of the leading causes of morbidity and mortality worldwide. Traditional treatments include surgery, chemotherapy and radiation therapy, and more recently targeted therapies including immunotherapy are becoming routine care for some cancers. Immunotherapy aims to upregulate the patient’s own immune system, enabling it to destroy cancerous cells. Obesity is a metabolic disorder characterized by significant weight that is an important contributor to many different diseases, including cancers. Obesity impacts the immune system and causes, among other things, a state of chronic low-grade inflammation. This is hypothesized to impact the efficacy of the immunotherapies, such as immune checkpoint inhibitors, although not necessarily in a negative way. Data from several studies show that even though obesity causes a state of chronic low-grade inflammation with reductions in effector immune populations, it has a beneficial effect on patient survival following anti-PD-1/PD-L1 and anti-CTLA-4 treatment.
- obesity
- metabolic syndrome
- cancer
- immunotherapy
- checkpoint therapy
- inflammation
- T-cell exhaustion
1. Cancer and Obesity
Cancer is a non-communicable disease brought about by changes within cells, resulting in their uncontrolled growth and division[1]. It is one of the leading causes of mortality worldwide, and most deaths attributed to cancer worldwide are from lung, breast, colorectal, stomach and liver cancers[2][3]. Furthermore, the number of new cancer registrations is increasing globally[2].
Generally defined by a Body Mass Index (BMI) of ≥30, obesity is caused by an energy imbalance that favours weight gain, resulting in metabolic disturbances causing stress to tissues and ultimately leads to disease[4]. According to World Health Organisation estimates, in 2016, 39% of adults aged 18 years or older were found to be overweight and 13% were obese globally. The prevalence of these conditions has risen in both adults and children[2]. The increasing prevalence of obesity has resulted in increasing morbidity and years of life lost due to cardiovascular disease, type-2 diabetes mellitus, osteoarthritis, psychological problems and obesity-related cancers[5]. Reports from the World Cancer Research Fund and the International Agency for Research into Cancer have found that several types of cancer are associated with obesity, specifically endometrial, oesophageal adenocarcinoma, colorectal, breast cancer in postmenopausal women, prostate and renal cancers. Overall, the number of cases of cancer estimated to be caused by obesity is 20% and obesity is the second highest risk factor for cancer, after tobacco smoking[6]. Furthermore, studies have found that obesity leads to poorer cancer treatment efficacy and greater mortality from cancer[6][7][8][9]. Many factors are attributed to this, such as difficulties in adjusting dose for chemotherapy and positioning obese patients for radiation therapy[10][11]. Morbidly obese (BMI > 35) as well as underweight (BMI < 18.5) patients also have higher mortality rates following curative cancer resection surgery compared to normal weight and overweight patients[12].
2. Obesity and the Immune System
The links between obesity and cancer have largely been related to the effects of insulin resistance, elevated sex hormones, modulation of adipokine secretion, and upregulation of Programmed Cell Death Protein (PD)-1 expression[7][13]. Obesity affects the immune system in a way that is relevant in both cancer progression and treatment, and the general hypothesis is that these changes will reduce the efficacy of immune-based treatments. The key ways that obesity and the immune system interact are outlined below.
2.1. Chronic Inflammation
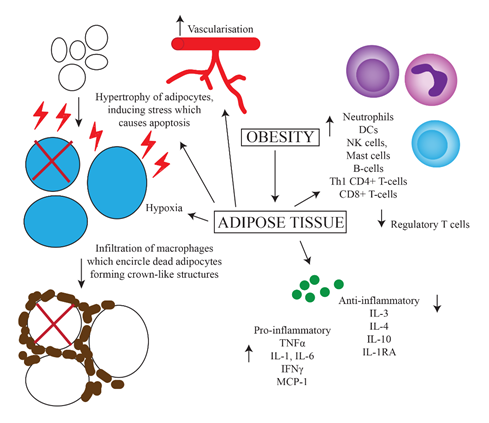
The obese state contributes to chronic inflammation by a number of mechanisms including adipocyte hypertrophy, macrophage recruitment and polarization, and increased production of pro-inflammatory mediators. Adipose tissue is required to expand in order to accommodate the influx of nutrients as seen in obesity[4]. In adults, adipocyte hypertrophy is favoured over hyperplasia[14]. These hypertrophic adipocytes induce shear mechanical stress on the extracellular environment and activate endoplasmic reticulum and mitochondrial stress responses. Overall, this results in a pro-inflammatory state within adipose tissue[15]. Figure 1 outlines the main contributors to this state.
The persistent state of inflammation and stress in adipose tissue leads to an increased expression of pro-apoptotic proteins, in particular Fas and its ligand, resulting in adipocyte cell death[16]. Following this, macrophages infiltrate the adipose tissue and encircle the dead adipocytes to form crown-like structures (CLS)[17][18]. CLS have been found in 50% of patients with breast cancer and their presence is associated with higher BMI and other systemic markers of metabolic syndrome. The formation of CLS causes the activation of pattern recognition receptors on macrophages, such as toll-like receptors (TLRs)[17]. As a result, macrophages are polarized towards a pro-inflammatory phenotype as opposed to an anti-inflammatory phenotype observed in healthy adipose tissue[19]. Other changes in the adipose tissue of obese individuals which drive inflammation include a reduction in the level of regulatory T-cells, increased fatty acid influx, vascularization, hypoxia, and increased leptin secretion[20]. Increases in proportions of neutrophils, dendritic cells (DCs), natural killer (NK) cells, mast cells, B-cells, Th1 CD4+ T-cells and CD8+ T-cells in the adipose tissue of obese individuals has also been shown[21]. One theory for this is an increased expression of Major Histocompatibility Complex (MHC)-II by adipocytes via a leptin-dependent mechanism, resulting in greater recruitment of leukocytes[21].
Both macrophages and hypertrophic adipocytes with increased intracellular stress upregulate secretion of the pro-inflammatory cytokines tumour necrosis factor (TNF)α, interleukin (IL)-1, IL-6, interferon (IFN)γ and monocyte chemoattractant protein-1[22][23]. As well as promoting inflammation, these mediators also block the production of adiponectin, which has anti-inflammatory effects. This expression of cytokines is higher in the more pathogenic visceral adipose tissue compared to subcutaneous adipose tissue[24]. Furthermore, the production of anti-inflammatory cytokines such as IL-3, IL-4, IL-10 and IL-1 receptor antagonist is decreased[23]. Molecules such as TNFα are also pro-angiogenic, and support the development of tumours[25]. Overall, this results in the development of insulin resistance, increased lipolysis and impaired lipid storage[4]. This change towards a basal pro-inflammatory state in obesity has been identified beyond adipose tissue, including in leukocytes circulating in the blood of obese people. These cells demonstrate greater nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation, which participates in the regulation of pro-inflammatory genes such as those involved in cytokine and chemokine production[26]. Furthermore, increased total lymphocyte, CD4+ and CD8+ T-cell and neutrophil counts have been associated with obesity[27][28][29]. However, several studies have failed to find consistent differences in the cytokine profile of non-obese and obese individuals that matches the current theories, although an increase in the pro-inflammatory cytokines IL-6 and TNFα have been observed[24][30][31][32]. This speaks to the complexity of the inflammatory changes brought about by obesity.
Figure 1. Predominant mechanisms of chronic inflammation caused by obesity. Increased uptake of nutrients leads to greater storage of fats and hence hypertrophy of adipocytes. This results in increased intracellular stress and upregulation of apoptotic genes, leading to apoptosis. Increased vascularization, hypoxia, cell death and upregulation of MHC-II on adipocytes leads to the influx of various inflammatory cells including macrophages, which surround dead adipocytes forming crown-like structures. There is also increased secretion of pro-inflammatory, and decreased secretion of anti-inflammatory cytokines.
2.2. Altered Production of Immune Cells
Mobilization of fat stores as a result of increased lipolysis and impaired lipid storage in adipose tissue causes an accumulation of lipids in non-adipose tissue, including lymphoid tissues like the bone marrow and thymus[33]. Bone marrow-derived hematopoietic stem cells are continuously replicating in order to maintain lymphoid (T- and B-lymphocytes, NK cells) and myeloid-derived (monocytes, macrophages, DCs, granulocytes, erythrocytes, megakaryocytes, mast cells) lineages of cells[34]. Immature T-cells then travel to and undergo further development in the thymus. Increased lipid deposits in the thymus and bone marrow, both primary lymphoid organs, disrupt their integrity, altering the environment in which leukocytes develop[35]. In the bone marrow, this suppresses haematopoiesis and skews progenitor populations into producing a greater ratio of myeloid progenitor cells as opposed to lymphoid progenitor cells[36][37].
In the thymus, changes occur which resemble the natural process of thymic involution that normally occurs with aging[35]. This includes a loss of corticomedullary junctions, increased perithymic adiposity, and a reduction in populations of lymphocytic precursor cells[37]. These changes result in a reduced thymic output of naïve T-cells, which is likely to negatively affect immune surveillance and therefore increase the likelihood of immune escape of pathogens or tumours[37].
2.3. Reduction of T-Cell Variation
Obesity has been linked to a reduction in the diversity of T-cell receptors (TCRs) on circulating T-cells, reducing the number of antigens that can be recognized and responded to[33]. Obesity has also been shown to cause a reduction in lymph node size, impair lymphatic fluid transport and migration of DCs to peripheral lymph nodes, and reduce the number of T-cells in the lymph nodes. These changes reduce the ability of the immune system to recognize and effectively deal with foreign antigens[15]. Furthermore, the expansion of adipocytes caused by obesity suppresses anti-inflammatory pathways, enabling DCs and T-cells to become activated within visceral white adipose tissue[17]. However, constant presentation of antigens by DCs may eventually lead to T-cell exhaustion and chronic inflammation, reducing the capability for T-cells to have a successful effector response[17].
3. Obesity and Immune Checkpoint Blockade
Antibodies neutralising inhibitory immune checkpoint molecules typically have an anti-cancer effect through targeting PD-1/PD-L1 and CTLA-4 receptors. This is important as the expression of PD-L1 in numerous cancer types including head and neck squamous cell carcinoma, lung carcinomas, endometrial, ovarian, breast cancers and melanoma has been shown to contribute to the evasion by these tumour cells from the immune system[38]. CTLA-4 expression has been implicated in immune dysregulation of cervical, breast, lung gastric, colorectal, skin, non-Hodgkin’s lymphoma and B-cell chronic leukaemia[39]. Combination therapies against both of these receptors used in patients with melanoma and non-small-cell lung carcinoma have been effective at increasing tumour remission and survival, and trials for these treatments against other types of cancer are underway[40][41][42][43].
Because of the link between obesity and the immune system, there has been increasing interest over the past few years into analysing the effect that obesity has on immune checkpoint therapies. A retrospective study found that patients with a variety of cancers, including melanoma, non-small cell lung cancer, and renal cell carcinoma who are classified as overweight or obese have been shown to have a better response to anti-PD-1/PD-L1 immune checkpoint inhibitors[44][45]. This finding was also replicated in studies where melanoma or lung tumour-bearing DIO mice had an increased response to anti-PD-1 treatment compared to lean mice, with decreased melanoma metastases also being observed. The improved efficacy in DIO mice was associated with a significantly increased tumour-infiltrating T-cell count, increased CD8:CD4 ratio, and increased frequency of CD8+ T-cells in the TME. These factors are all considered to be correlated with positive outcomes[46][47][48]. The frequency of PD-1+ T-cells in the TME was also reduced in DIO mice after anti-PD-1 therapy, signifying an increased rate of T-cells which had been rescued from an exhausted state[49]. Increased expression of PD-1 by T-cells, is one of the potential reasons why obese mice and humans have stronger responses to anti-PD-1/PD-L1 treatment[49].
Several retrospective studies have confirmed the association of improved survival with increased weight in people with advanced/metastatic melanoma[50][51]. These studies found increased overall survival in overweight patients treated with either anti-PD-1/PD-L1 or anti-PD-1 + anti-CTLA-4 therapy. The association was predominantly found in males who had high serum creatinine levels (a marker for high muscle mass)[51]. Another study found that obese patients had a statistically significant improvement in progression-free survival (PFS) when treated with anti-PD-1 or anti-CTLA-4, although there was no improvement overall[52]. A separate study found a linear association between increased BMI and overall survival in patients with non-small cell lung cancer (NSCLC) treated with atezolizumab (anti-PD-L1). This association between BMI and survival was not found the control group who were treated with the chemotherapy agent, docetaxel. In particular, patients with a BMI ≥ 30 had significantly improved overall survival. Adverse events were not associated with differences in BMI[53]. Tables 1 and 2 summarise studies investigating the effects of obesity on cancer immunotherapy outcomes.
Table 1. Human studies published before February 2020 investigating the effects of obesity on immune checkpoint blockade therapy for cancer.
Study Authors |
Date of Study |
Type of Study |
Cancer |
Drug Name |
Statistical Effects of Obesity |
|
Human trials |
|||||
|
Cortellini et al.[45] |
February 2019 |
Retrospective |
NSCLC, melanoma, renal cell carcinoma, others |
Anti-PD-1/PD-L1 (pembrolizumab, nivolumab or atezolizumab) |
Objective response rate, time to treatment failure (HR = 0.51 [95% CI: 0.44–0.60], progress-free survival (HR = 0.46 [95%CI: 0.39–0.54]) and overall survival (HR = 0.33 [95%CI: 0.28–0.41]), significantly improved in overweight/obese patients (p < 0.0001) |
|
Donnelly et al.[52] |
August 2019 |
RCT |
Metastatic melanoma |
Anti-PD-1/anti-CTLA-4 (specific drugs unspecified) |
No difference in PFS or OS between BMI levels in monotherapy however PFS for combination therapy was significant in obese patients (HR = 0.17 [95%CI: 0.04–0.65]) (p = 0.02) |
|
Kichenadasse et al.[53] |
December 2019 |
RCT |
Non-small cell lung cancer |
Atezolizumab (anti-PD-L1) |
BMI ≥ 30 increase in OS (HR = 0.36 [95%CI: 0.21–0.62]) (p < 0.001) |
|
McQuade et al.[50] |
February 2018 |
Retrospective |
Metastatic melanoma |
Anti-PD-1/PD-L1, ipilimumab+ dacarbazine |
Anti-PD-1/PD-L1: increased PFS (HR = 0.69 [95%CI: 0.45–1.06]) and OS (HR = 0.69 [95%CI: 0.42–1.12] for overweight and obese male patients compared to normal weight patients (not statistically significant), but not for female patients Anti-CTLA-4: increased PFS (HR = 0.55 [95%CI: 0.32–0.93]) and OS (HR = 0.40 [95%CI: 0.22–0.72]) in obese male patients compared to normal weight patients (not statistically significant), but not for female patients |
|
Naik et al.[51] |
March 2019 |
Retrospective |
Unresectable or metastatic melanoma |
Pembrolizumab or nivolumab (anti-PD-1) or anti-PD-1+ ipilimumab (anti-CTLA-4) |
Overweight (but not obese) patients had increased OS compared to normal weight patients (HR = 0.26 [95%CI: 0.1–0.71]) (p = 0.038) |
|
Richtig et al.[60] |
October 2018 |
Retrospective |
Metastatic melanoma |
Anti-CTLA4 (ipilimumab) |
Overweight and obese patients have higher response rates (p = 0.024, no other statistics provided) and a lower likelihood of brain metastases (8.6% vs 32.5%, p = 0.012) compared to normal weight patients, as well as longer overall survival (HR = 1.81 [95%CI: 0.98–3.33], p = 0.056) |
|
Wang et al.[54] |
November 2018 |
RCT |
Lung cancer, melanoma, ovarian cancer, and others (unspecified) |
Anti-PD-L1/ |
Improvement in progression free survival (median: 237 vs 141 days, p = 0.0034) and overall survival (median: 523 vs 361 days, p = 0.0492) in obese (BMI > 30) compared to non-obese (BMI < 30) patients |
HR = hazard ratio, CI = confidence interval, OS = overall survival, PFS = progression-free survival.
Table 2. The effects of obesity on immune checkpoint blockade therapy for cancer trialed in animal studies.
Study Authors |
Date of Study |
Type of Study |
Cancer |
Drug Name |
Statistical Effect of Obesity |
|
Animal trials |
|||||
|
Murphy et al.[61] |
August 2018 |
Tumour trial |
Renca (renal adenocarcinoma) |
Anti-CTLA-4 |
Compared to control, increased survival in lean mice (p = 0.007) and ob/ob mice (p = 0.005) but not DIO mice (p = 0.095), no other statistics provided |
|
Wang et al.[54] |
November 2018 |
Tumour trial |
B16 (melanoma) |
Anti-PD-1 |
DIO mice have reduced tumour growth by day 16 (p < 0.005), no other statistics provided |
|
Wang et al.[54] |
November 2018 |
Tumour trial |
3ll (lung cancer) |
Anti-PD-1 |
DIO mice have reduced tumour growth by day 11 (p < 0.001), no other statistics provided |
Fewer studies have looked at the effect of obesity on anti-CTLA-4 treatment. A retrospective study found that patients with metastatic melanoma, who were treated with ipilimumab as a monotherapy, had significantly increased response rates when patients had a BMI ≥ 25 (either overweight or obese) compared to those with a BMI < 25 (normal or underweight)[60]. No differences were found between gender or immune-related adverse effects. Overweight and obese patients also had a lower rate of brain metastases, and a trend of longer overall survival times. Another study also found a trend of increased overall survival and progression-free survival in obese males compared to normal weight males, but not females[50]. In contrast, a murine study looking at the effects of obesity on anti-CTLA-4 treatment of adenocarcinoma found reduced efficacy in obese BALB/c mice[61]. Lean mice and ob/ob (leptin deficient obese) mice had improved overall survival after anti-CTLA-4 therapy compared to the PBS control. However, DIO mice did not respond to treatment. One reason for this is thought to be the high levels of leptin in the DIO mice. When leptin levels were reduced, the ability to respond to treatment was returned, inferring a potential inverse relationship between leptin concentration and CTLA-4 expression. However, there is limited research into the link between leptin and CTLA-4 in the context of cancer and no potential mechanisms have been proposed.
Because obesity is a multi-faceted disease, it is likely that several pathways contribute to the observed clinical benefit of obesity on immune checkpoint blockade therapy. While no biological link has been confirmed yet, one proposed mechanism is that the increased expression of PD-1 triggered by heightened leptin levels is responsible for this phenomenon.
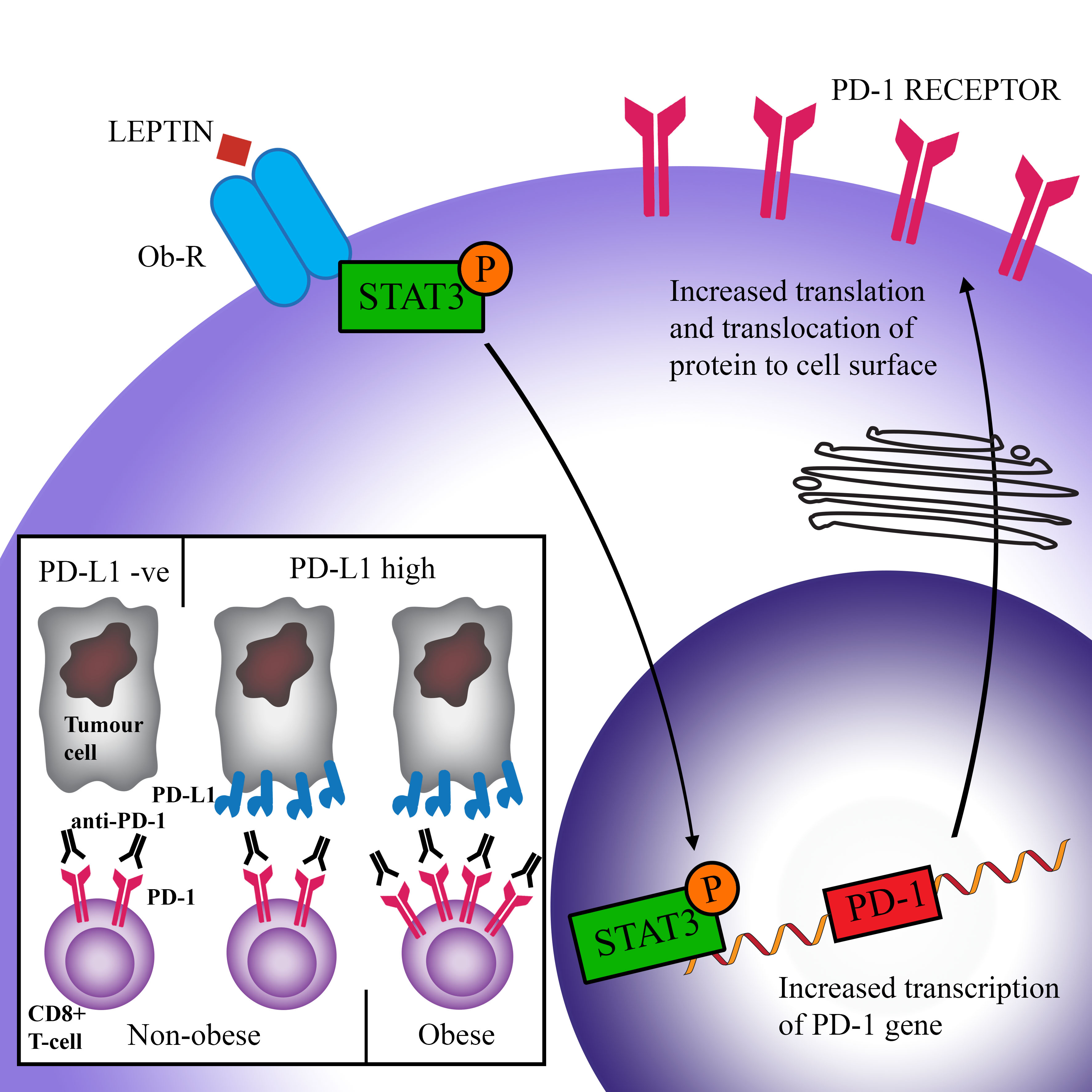
Wang et al. hypothesized that the increased expression of PD-1 in obese animals may be due to the raised levels of leptin as seen in obesity[54]. This hormone is produced by adipose tissue and acts to suppress food intake[55]. Many obese people develop leptin resistance, either due to the impaired transport of leptin across the blood–brain barrier, or problems downstream of the leptin signal. As a result, obese people often have high levels of leptin[56][57]. Signal transducer and activator of transcription (STAT)3, which is a major downstream transcription factor of the leptin receptor, can bind to the promoter region of PD-1 and cause the transcription and subsequent translation of this protein[58][59]. Stimulation of CD8+ T-cells by leptin has been shown to upregulate STAT3 and was correlated with increased expression of PD-1[54]. Figure 2 shows a schematic representation of this hypothesis. Furthermore, increased levels of STAT3 have been associated with the expression of PD-1 in many cancers[54]. While elevated PD-1 expression is related to increased T-cell exhaustion and therefore reduced T-cell proliferation and function, it also means that therapies that target PD-1 have improved efficacy.
It is therefore conceivable that with increased expression of PD-1 on the surface of immune cells, the interaction between PD-1 and PD-L1 (on tumour cells) is increased, thus impairing anti-cancer immune responses. Anti-PD-1/PD-L1 therapy, which inhibits this interaction and allows CD8+ T-cells to have increased ability to kill tumour cells, would therefore be more efficacious (Figure 2). This theory is supported by the study by Kichenadasse et al., who found that the association between obesity and immune checkpoint blockade success was strongest in patients with a higher expression of PD-L1, while there was no difference in survival in patients with PD-L1 negative cancers[53]. This shows that checkpoint therapy can only be effective if the ligands for checkpoint molecules are expressed.
Figure 2. Scheme of a proposed pathway causing increased efficacy of immune checkpoint blockade in obese patients. Leptin binds to its receptor (Ob-R) on CD8+ T-cells and causes the activation (via phosphorylation) of the transcription factor STAT3. STAT3 triggers the transcription of the PD-1 gene and subsequent expression of the PD-1 protein on the cell surface. Higher PD-1 levels are correlated with increased exhaustion (activation by PD-L1 reduces T-cell proliferation, survival, and production of cytokines). However, increased PD-1 expression facilitates greater success of anti-PD-1 therapy, leading to increased overall survival in obese patients.
One concern with administering immunotherapy in obese patients was the risk of an increase in immune-related adverse effects. For instance, it was found that the administration of anti-CD40/IL-2 immunotherapy into leptin-deficient obese mice resulted in a ‘cytokine storm’, with high levels of TNFα and IL-6 being released, causing multi-organ pathological responses and rapid lethality. This has been attributed to the baseline level of chronic inflammation found in the obese mice[62]. To date, this has rarely been observed in obese patients nor in preclinical mouse models[54][53][49]. One study did find that overweight and obese patients were twice as likely to suffer from immune-related adverse events, although not from higher grade adverse effects[45]. This study found, however, that an increase in these events was independently associated with increased efficacy of treatment[45].
This entry is adapted from the peer-reviewed paper 10.3390/cancers12051230
References
- Cooper, G.M.. The development and causes of cancer; Sinauer Associates: Sunderland, MA, USA, 2000; pp. x.
- Cancer 2018 Fact Sheet . World Health Organisation. Retrieved 2020-11-6
- New Cancer Registrations . Ministry of Health. Retrieved 2020-11-6
- Adilson Guilherme; Joseph V. Virbasius; Vishwajeet Puri; Michael P. Czech; Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature Reviews Molecular Cell Biology 2008, 9, 367-377, 10.1038/nrm2391.
- John B. Dixon; The effect of obesity on health outcomes. Molecular and Cellular Endocrinology 2010, 316, 104-108, 10.1016/j.mce.2009.07.008.
- Kathleen Y. Wolin; Kenneth Carson; Graham A. Colditz; Obesity and Cancer. The Oncologist 2010, 15, 556-565, 10.1634/theoncologist.2009-0285.
- Giovanni De Pergola; Franco Silvestris; Obesity as a Major Risk Factor for Cancer. Journal of Obesity 2013, 2013, 1-11, 10.1155/2013/291546.
- Gillian K Reeves; Kirstin Pirie; Valerie Beral; Jane Green; Elizabeth Spencer; Diana Bull; Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007, 335, 1134-1134, 10.1136/bmj.39367.495995.ae.
- Ivana Vucenik; Joseph P. Stains; Obesity and cancer risk: evidence, mechanisms, and recommendations. Annals of the New York Academy of Sciences 2012, 1271, 37-43, 10.1111/j.1749-6632.2012.06750.x.
- Neil S. Horowitz; Alexi A. Wright; Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecologic Oncology 2015, 138, 201-206, 10.1016/j.ygyno.2015.04.002.
- Małgorzata Moszyńska-Zielińska; Justyna Chałubińska-Fendler; Leszek Gottwald; Leszek Żytko; Ewelina Bigos; Jacek Fijuth; Does obesity hinder radiotherapy in endometrial cancer patients? The implementation of new techniques in adjuvant radiotherapy – focus on obese patients. Menopausal Review 2014, 2, 96-100, 10.5114/pm.2014.42710.
- Candyce H. Kroenke; Romain Neugebauer; Jeffrey Meyerhardt; Carla M. Prado; Erin Weltzien; Marilyn L. Kwan; Jingjie Xiao; Bette J. Caan; Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams.. JAMA Oncology 2016, 2, 1137-45, 10.1001/jamaoncol.2016.0732.
- Timothy F. Cloughesy; Aaron Y. Mochizuki; Joey R. Orpilla; Willy Hugo; Alexander H. Lee; Tom B. Davidson; Anthony C. Wang; Benjamin M. Ellingson; Julie A. Rytlewski; Catherine M. Sanders; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nature Medicine 2019, 25, 477-486, 10.1038/s41591-018-0337-7.
- Afia Naaz; Denise R. Holsberger; Gary A. Iwamoto; Amanda Nelson; Hiroaki Kiyokawa; Paul S. Cooke; Loss of cyclin‐dependent kinase inhibitors produces adipocyte hyperplasia and obesity. The FASEB Journal 2004, 18, 1925-1927, 10.1096/fj.04-2631fje.
- Catherine J Andersen; Kelsey E Murphy; Maria Luz Fernandez; Impact of Obesity and Metabolic Syndrome on Immunity. Advances in Nutrition 2016, 7, 66-75, 10.3945/an.115.010207.
- Zsuzsa Szondy; Zsolt Sarang; Beáta Kiss; Éva Garabuczi; Krisztina Köröskényi; Anti-inflammatory Mechanisms Triggered by Apoptotic Cells during Their Clearance. Frontiers in Immunology 2017, 8, 909, 10.3389/fimmu.2017.00909.
- Daniela F. Quail; Andrew J. Dannenberg; The obese adipose tissue microenvironment in cancer development and progression. Nature Reviews Endocrinology 2018, 15, 139-154, 10.1038/s41574-018-0126-x.
- Peter Sartipy; David J. Loskutoff; Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proceedings of the National Academy of Sciences 2003, 100, 7265-7270, 10.1073/pnas.1133870100.
- Carey N. Lumeng; Jennifer L. Bodzin; Alan R. Saltiel; Obesity induces a phenotypic switch in adipose tissue macrophage polarization. Journal of Clinical Investigation 2007, 117, 175-184, 10.1172/jci29881.
- Bonnie K Surmi; Alyssa H Hasty; Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future Lipidology 2008, 3, 545-556, 10.2217/17460875.3.5.545.
- A. W. Ferrante; The immune cells in adipose tissue. Diabetes, Obesity and Metabolism 2013, 15, 34-38, 10.1111/dom.12154.
- C. Lagathu; L. Yvan-Charvet; J.-P. Bastard; M. Maachi; A. Quignard-Boulangé; J. Capeau; M Caron; Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia 2006, 49, 2162-2173, 10.1007/s00125-006-0335-z.
- Hansongyi Lee; In Seok Lee; Ryowon Choue; Obesity, Inflammation and Diet. Pediatric Gastroenterology, Hepatology & Nutrition 2013, 16, 143-152, 10.5223/pghn.2013.16.3.143.
- Volatiana Rakotoarivelo; Gregory Lacraz; Marian Mayhue; Christine Brown; Diane Rottembourg; Julie Fradette; Subburaj Ilangumaran; Alfredo Menendez; Marie-France Langlois; Sheela Ramanathan; et al. Inflammatory Cytokine Profiles in Visceral and Subcutaneous Adipose Tissues of Obese Patients Undergoing Bariatric Surgery Reveal Lack of Correlation With Obesity or Diabetes. EBioMedicine 2018, 30, 237-247, 10.1016/j.ebiom.2018.03.004.
- Sharath P. Sasi; Xinhua Yan; Heiko Enderling; Daniel S. Park; Hui-Ya Gilbert; Cindy Curry; Christina Coleman; Lynn Hlatky; Gangjian Qin; Raj Kishore; et al. Breaking the 'harmony' of TNF-α signaling for cancer treatment.. Oncogene 2011, 31, 4117-27, 10.1038/onc.2011.567.
- Husam Ghanim; Ahmad Aljada; Deborah Hofmeyer; Tufail Syed; Priya Mohanty; Paresh Dandona; Circulating Mononuclear Cells in the Obese Are in a Proinflammatory State. Circulation 2004, 110, 1564-1571, 10.1161/01.cir.0000142055.53122.fa.
- Y Furuncuoğlu; S Tulgar; A N Dogan; S Cakar; Y K Tulgar; B Cakiroglu; How obesity affects the neutrophil/lymphocyte and platelet/lymphocyte ratio, systemic immune-inflammatory index and platelet indices: a retrospective study.. European Review for Medical and Pharmacological Sciences 2016, 20, 1300-1306, .
- Blanche C. Ip; Andrew E. Hogan; Barbara S. Nikolajczyk; Lymphocyte roles in metabolic dysfunction: of men and mice.. Trends in Endocrinology & Metabolism 2015, 26, 91-100, 10.1016/j.tem.2014.12.001.
- Julie A. Womack; Phyllis C. Tien; Joseph Feldman; Ja Hyun Shin; Kristopher P. Fennie; Kathryn Anastos; Mardge H. Cohen; Melanie C. Bacon; Howard Minkoff; Obesity and immune cell counts in women. Metabolism 2007, 56, 998-1004, 10.1016/j.metabol.2007.03.008.
- Bashir Ahmad Laway; Hamid Ashraf; Dil Afroze; Arshad Iqbal Wani; Evaluation of proinflammatory cytokines in obese vs non-obese patients with metabolic syndrome. Indian Journal of Endocrinology and Metabolism 2018, 22, 751-756, 10.4103/ijem.IJEM_206_18.
- Maryam Azizian; Elahe Mahdipour; Seyed Reza Mirhafez; Sara Shoeibi; Mohsen Nematy; Habibollah Esmaily; Gordon Aa Ferns; Majid Ghayour Mobarhan; Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Annals of Clinical Biochemistry: An international journal of biochemistry and laboratory medicine 2016, 53, 663-668, 10.1177/0004563216629997.
- Ethan G. Aguilar; William J. Murphy; Obesity induced T cell dysfunction and implications for cancer immunotherapy. Current Opinion in Immunology 2018, 51, 181-186, 10.1016/j.coi.2018.03.012.
- Thirumala-Devi Kanneganti; Vishwa Deep Dixit; Immunological complications of obesity. Nature Immunology 2012, 13, 707-712, 10.1038/ni.2343.
- Hiromi Iwasaki; Koichi Akashi; Myeloid Lineage Commitment from the Hematopoietic Stem Cell. Immunity 2007, 26, 726-740, 10.1016/j.immuni.2007.06.004.
- Vishwa Deep Dixit; Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Seminars in Immunology 2012, 24, 321-330, 10.1016/j.smim.2012.04.002.
- Olaia Naveiras; Valentina Nardi; Pamela L. Wenzel; Peter V. Hauschka; Frederic H Fahey; George Q. Daley; Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259-263, 10.1038/nature08099.
- Hyunwon Yang; Yun-Hee Youm; Bolormaa Vandanmagsar; Jennifer Rood; K. Ganesh Kumar; Andrew A. Butler; Vishwa Deep Dixit; Obesity accelerates thymic aging. Blood 2009, 114, 3803-3812, 10.1182/blood-2009-03-213595.
- Nyanbol Kuol; Lily Stojanovska; Kulmira Nurgali; Vasso Apostolopoulos; The mechanisms tumor cells utilize to evade the host's immune system. Maturitas 2017, 105, 8-15, 10.1016/j.maturitas.2017.04.014.
- Pingping Hu; QiQi Liu; Guodong Deng; Jingxin Zhang; Ning Liang; Jian Xie; Jiandong Zhang; The prognostic value of cytotoxic T-lymphocyte antigen 4 in cancers: a systematic review and meta-analysis. Scientific Reports 2017, 7, srep42913, 10.1038/srep42913.
- Jedd D. Wolchok; Vanna Chiarion-Sileni; Rene Gonzalez; P. Rutkowski; Jean Jacques Grob; C. Lance Cowey; Christopher D. Lao; John Wagstaff; Dirk Schadendorf; Pier F. Ferrucci; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New England Journal of Medicine 2017, 377, 1345-1356, 10.1056/nejmoa1709684.
- James Larkin; Vanna Chiarion-Sileni; Rene Gonzalez; Jean-Jacques Grob; Piotr Rutkowski; Christopher D. Lao; C. Lance Cowey; Dirk Schadendorf; John Wagstaff; Reinhard Dummer; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New England Journal of Medicine 2019, 381, 1535-1546, 10.1056/nejmoa1910836.
- James Larkin; Vanna Chiarion-Sileni; Rene Gonzalez; Jean Jacques Grob; C. Lance Cowey; Christopher D. Lao; Dirk Schadendorf; Reinhard Dummer; Michael Smylie; Piotr Rutkowski; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New England Journal of Medicine 2015, 373, 23-34, 10.1056/nejmoa1504030.
- Matthew D. Hellmann; Luis Paz-Ares; Reyes Bernabe Caro; Bogdan Zurawski; Sang-We Kim; Enric Carcereny Costa; Keunchil Park; Aurelia Alexandru; Lorena Lupinacci; Emmanuel De La Mora Jimenez; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. New England Journal of Medicine 2019, 381, 2020-2031, 10.1056/nejmoa1910231.
- Flora Sánchez-Jiménez; Antonio Pérez-Pérez; Luis De La Cruz-Merino; Víctor Sánchez-Margalet; Obesity and Breast Cancer: Role of Leptin. Frontiers in Oncology 2019, 9, 596, 10.3389/fonc.2019.00596.
- Alessio Cortellini; Melissa Bersanelli; Sebastiano Buti; Katia Cannita; Daniele Santini; Fabiana Perrone; Raffaele Giusti; Marcello Tiseo; Maria Michiara; Pietro Di Marino; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. Journal for ImmunoTherapy of Cancer 2019, 7, 57, 10.1186/s40425-019-0527-y.
- Qian-Kun Xie; Wen-Zhuo He; Wan-Ming Hu; Lin Yang; Chang Jiang; Peng-Fei Kong; Yuan-Zhong Yang; Qiong Yang; Hui-Zhong Zhang; Bei Zhang; et al. Tumor-infiltrating lymphocyte as a prognostic biomarker in stage IV colorectal cancer should take into account the metastatic status and operation modality. Cancer Management and Research 2018, 10, 1365-1375, 10.2147/CMAR.S162147.
- Shota Shimizu; Hiroyoshi Hiratsuka; Kazushige Koike; Kei Tsuchihashi; Tomoko Sonoda; Kazuhiro Ogi; Akira Miyakawa; Junichi Kobayashi; Takeshi Kaneko; Tomohiro Igarashi; et al. Tumor-infiltrating CD8+ T-cell density is an independent prognostic marker for oral squamous cell carcinoma. Cancer Medicine 2019, 8, 80-93, 10.1002/cam4.1889.
- Rathore, Ankita Singh; Kumar, Sandeep; Konwar, Rituraj; Makker, Annu; Negi, M.P.S.; Goel, Madhu Mati; CD3+, CD4+ & CD8+ tumour infiltrating lymphocytes (TILs) are predictors of favourable survival outcome in infiltrating ductal carcinoma of breast. Indian Journal of Medical Research 2014, 140, 361-369, .
- William J. Murphy; Dan L. Longo; The Surprisingly Positive Association Between Obesity and Cancer Immunotherapy Efficacy. JAMA 2019, 321, 1247-1248, 10.1001/jama.2019.0463.
- Jennifer L. McQuade; Carrie R. Daniel; Kenneth R. Hess; Carmen Mak; Daniel Y. Wang; Rajat R. Rai; John J. Park; Lauren E. Haydu; Christine Spencer; Matthew Wongchenko; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. The Lancet Oncology 2018, 19, 310-322, 10.1016/s1470-2045(18)30078-0.
- Girish S. Naik; Sushrut S. Waikar; Alistair E. W. Johnson; Elizabeth I. Buchbinder; Rizwan Haq; F. Stephen Hodi; Jonathan D. Schoenfeld; Patrick A. Ott; Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. Journal for ImmunoTherapy of Cancer 2019, 7, 89, 10.1186/s40425-019-0512-5.
- Douglas Donnelly; Shirin Bajaj; Jaehong Yu; Miles Hsu; Arjun Balar; Anna Pavlick; Jeffrey Weber; Iman Osman; Judy Zhong; The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. Journal for ImmunoTherapy of Cancer 2019, 7, 222, 10.1186/s40425-019-0699-5.
- Ganessan Kichenadasse; John O. Miners; Arduino A. Mangoni; Andrew Rowland; Ashley M. Hopkins; Michael J. Sorich; Association Between Body Mass Index and Overall Survival With Immune Checkpoint Inhibitor Therapy for Advanced Non–Small Cell Lung Cancer. JAMA Oncology 2020, 6, 512, 10.1001/jamaoncol.2019.5241.
- Ziming Wang; Ethan G. Aguilar; Jesus I. Luna; Cordelia Dunai; Lam T. Khuat; Catherine T. Le; Annie Mirsoian; Christine M. Minnar; Kevin M. Stoffel; Ian R. Sturgill; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nature Medicine 2018, 25, 141-151, 10.1038/s41591-018-0221-5.
- Jeffrey Friedman; Leptin and the endocrine control of energy balance. Nature Metabolism 2019, 1, 754-764, 10.1038/s42255-019-0095-y.
- William A. Banks; Catherine L. Farrell; Impaired transport of leptin across the blood-brain barrier in obesity is acquired and reversible. American Journal of Physiology-Endocrinology and Metabolism 2003, 285, E10-E15, 10.1152/ajpendo.00468.2002.
- Heike Münzberg; Martin G Myers Jr; Martin G Myers; Molecular and anatomical determinants of central leptin resistance. Nature Neuroscience 2005, 8, 566-570, 10.1038/nn1454.
- James W. Austin; Peiyuan Lu; Parimal Majumder; Rafi Ahmed; Jeremy M. Boss; STAT3, STAT4, NFATc1, and CTCF Regulate PD-1 through Multiple Novel Regulatory Regions in Murine T Cells. The Journal of Immunology 2014, 192, 4876-4886, 10.4049/jimmunol.1302750.
- Sarah H. Bates; Walter H. Stearns; Trevor A. Dundon; Markus Schubert; Annette W. K. Tso; Yongping Wang; Alexander S. Banks; Hugh J. Lavery; Asma K. Haq; Eleftheria Maratos-Flier; et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 2003, 421, 856-859, 10.1038/nature01388.
- Georg Richtig; Christoph Hoeller; Martin Wolf; Ingrid Wolf; Barbara M. Rainer; Günter Schulter; Markus Richtig; Martin R. Grübler; Anna Gappmayer; Thomas Haidn; et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: An observational multi-centre study. PLOS ONE 2018, 13, e0204729, 10.1371/journal.pone.0204729.
- Katherine A. Murphy; Britnie R. James; Frances V. Sjaastad; Tamara A. Kucaba; Hyunjoon Kim; Erik L. Brincks; Streamson C. Chua; Andrew Wilber; Thomas S. Griffith; Cutting Edge: Elevated Leptin during Diet-Induced Obesity Reduces the Efficacy of Tumor Immunotherapy. The Journal of Immunology 2018, 201, 1837-1841, 10.4049/jimmunol.1701738.
- Annie Mirsoian; Myriam N. Bouchlaka; Gail D. Sckisel; Mingyi Chen; Chien-Chun Steven Pai; Emanuel Maverakis; Richard G. Spencer; Kenneth W. Fishbein; Sana Siddiqui; Arta M. Monjazeb; et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. Journal of Experimental Medicine 2014, 211, 2373-2383, 10.1084/jem.20140116.