Technological development is in constant progress in the oncological field. The search for new concepts and strategies for improving cancer diagnosis, treatment and outcomes constitutes a necessary and continuous process, aiming at more specificity, efficiency, safety and better quality of life of the patients throughout the treatment. Nanotechnology embraces these purposes, offering a wide armamentarium of nanosized systems with the potential to incorporate both diagnosis and therapeutic features, towards real-time monitoring of cancer treatment. Within the nanotechnology field, magnetic nanosystems stand out as complex and promising nanoparticles with magnetic properties, that enable the use of these constructs for magnetic resonance imaging and thermal therapy purposes. Additionally, magnetic nanoparticles can be tailored for increased specificity and reduced toxicity, and functionalized with contrast, targeting and therapeutic agents, revealing great potential as multifunctional nanoplatforms for application in cancer theranostics. This review aims at providing a comprehensive description of the current designs, characterization techniques, synthesis methods, and the role of magnetic nanoparticles as promising nanotheranostic agents. A critical appraisal of the impact, potentialities and challenges associated with each technology is also presented.
- cancer
- therapy
- diagnosis
- magnetic nanoparticles
- nanotheranostics
- biomedical applications
- drug delivery
1. Introduction
MNPs have demonstrated potentialities for diagnosis and therapeutics. In the context of diagnosis, these nanosized ensembles can be designed to perform as contrast agents in MRI, Positron Emission Tomography (PET), Computed Tomography (CT), Single Photon Emission Tomography (SPECT), Photoacoustic Imaging (PAI) and Surface Enhanced Raman Spectroscopy (SERS). On the other hand, MNPs for therapeutic applications may serve as drug delivery agents, gene delivery agents and/or thermoablation agents tuned for magnetic hyperthermia, Photothermal Therapy (PTT) and/or Photodynamic Therapy (PDT)[1].
2. Medical Applications
Magnetic resonance imaging is a non-invasive diagnostic tool that uses a magnetic field and radiofrequency electromagnetic pulse waves to provide a high spatial resolution of internal structures of the body. The imaging technique enables the detection of the variation on the direction of the rotation axis of the protons of water molecules, resulting in soft tissue contrast imaging and, as such, for contrast enhancement, requires the use of agents that affect the protons magnetic relaxation. Contrast enhancement is promoted by T1 or T2 contrast agents. T1 contrast agents, such as gadolinium, Gd (III), reduce longitudinal relaxation time and generate a brighter signal. T2 contrast agents, such as superparamagnetic nanoparticles (e.g., SPIONs), generate local magnetic fields that interfere and reduce the transverse relaxation time, resulting in a darker signal. Spherical nanocomposites combining both contrast agents, dextran-coated SPIONs with Gd ions inserted in the external MNP structure, have been synthesized by co-precipitation with Gd (III) nitrate for dual-modal imaging[2]. The Gd-doped system presented a nano-sized platform not only for MRI but also for hyperthermia therapy, making use of the relaxation energy loss in the form of heat to induce cell damage, that is intended to address the tumour tissue[2]. Further investigation would be required to produce MNPs with targeting moieties to direct the nanotheranostic agent to the target site and avoid negative interference and damage of healthy body cells and tissues. CT comprises an X-ray-based measurements technique that provides a cross-sectional X-ray imaging construct of the determined area of the body. It is a faster, less expensive method and provides images of tissues, organs and skeletal structure when compared to MRI, however, the latter represents a more advantageous method, due to the absence of the exposure to ionizing radiations. Alternatively, gold nanorods and spherical nanoparticles have been studied as contrast agents for the mentioned technique, due to the significant x-ray attenuation (with relevant x-ray scattering and absorption phenomena), when compared to normal tissue; higher proton attenuation coefficients, compared to iodine (the most commonly used contrast agent); and reduced toxicity. The particularity of these NPs is the potential association with IONPs for dual imaging integrating MRI and CT techniques[3].
PET is a highly sensitive molecular imaging that detects the radiation activity of positron emission radioisotopes, such as copper 64Cu2+, gallium 67/68Ga3+, indium 111In3+, fluorine 18F−, among others. MNPs designed for PET imaging require the formation of a complex with the selected radioisotope. Thus, macrocyclic chelating agents, such as 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetracetic acid (DOTA) are commonly used, since they tend to originate highly stable complexes, preventing transchelation events[4]. The obtained MNP loaded with the active radioisotope undergoes biodistribution in the body, accumulating in the highly chemically active areas/tissues and resulting in a bright signal that is detected by the equipment.
Similar to PET scan, single photon emission tomography (SPECT) exhibits high molecular sensitivity and reduced spatial resolution. Thus, the combination of either of the prementioned methods with a spatial resolution imaging technique would address and bridge their limitations and provide an anatomical and functional tool for diagnostic purposes.
Photoacoustic Imaging (PAI) is a hybrid imaging modality that combines optical and ultrasound imaging. The ultrasound imaging provides a high spatial resolution and the optical imaging delivers high contrast based on the tissue optical absorption[5]. The combination of both imaging techniques offers more information, greater specificity and penetration depth. Furthermore, PAI may provide a non-invasive method for the determination of the temperature distribution in tissues, as a relevant approach for monitoring of photothermal cancer therapies with highly laser light-absorbing contrast agents, such as gold-coated MNPs[5].
SERS comprises a vibrational spectroscopy technique of molecular signal amplification, with potential for in vivo imaging[6]. SERS uses the inelastic light scattering of photons that interact with matter, such as MNPs, to retrieve information about the surface characteristics and components. However, the technique is restricted to materials with high SERS properties, such as silver, gold and copper, and requires the preservation of these properties for in vivo cancer imaging, which has been reported as limited, when incorporated in theranostic nanoplatforms[7][8]. Li et al.[7] describe the design and synthesis of gold shell-core IONP (nanoflower-shaped NPs) with a rough surface for enhanced light scattering phenomena. The designed MNPs provided multimodal imaging application- SERS sensitivity, precise PA imaging and defined spatial resolution in MRI- and photothermal therapy performance, having expressed significantly elevated temperatures (dose-dependent temperature elevations of around 17 and 49 °C) for tumour ablation.
Regarding the therapeutic approaches, hyperthermia corresponds to one of the most explored. For the thermoablation procedure, various techniques have been implemented, such as laser, microwaves and ionizing radiation. However, the induced interference in the genetic material and low therapy selectivity have been identified as important side effects that can lead to healthy cell damage. Thus, the MNP-induced magnetic hyperthermia (MHT) provides an externally controllable local heating directed to a determined region of the body, which reduces the risk of damage of healthy tissues, in comparison to the other techniques mentioned above[9].
PTT is also a thermoablation technique that uses an infrared laser to activate light-absorbing MNPs, resulting in a higher heating capacity per nanosystem, when compared to magnetic hyperthermia. However, the incident infrared light penetration capacity is regarded as the most relevant of the technique limitations. Thus, the combination of both thermal therapies in a single MNP constitutes a beneficial merged therapeutic tool, that not only responds to the externally applied magnetic field, but also to the incident light[10].
A tri-modal therapy can also be integrated in a single nanosystem, by MNP tuning for MHT, PTT and Photodynamic Therapy (PDT). The latter technique involves the incidence of selective wavelength radiation that will induce the excitation state of a photosensitizer, which will transfer the energy to the surrounding oxygen molecules and originate the production of ROS and consequent cell death. A nanohybrid system composed of a multicore IONPs and a copper sulfide shell was produced using the polyol method and described as an optimized nanotheranostics platform with MRI and MHT responsive core, and PTT and PDT responsive shell. The integrated tri-modal thermal therapy nanoplatform conjugates an interesting system of cumulative heating capacity, that can provide a beneficial low dose approach in cancer nanotheranostics[10].
Magnetic nanoparticles can also serve as drug delivery systems, providing an extended surface area for drug loading and an optimized bioavailability, associated with lower drug administration doses and increased tissue selectivity[11]. Furthermore, the incorporation of chemotherapeutic agents in these nano-sized platforms can be achieved by the previous coating with agents that provide coupling points for conjugation, complexation or encapsulation of the selected drug. The MNPs loading capacity for anti-cancer drugs has been studied, being doxorubicin (DOX) the most widely used chemotherapeutic agent, with described application in various cancers, such as breast, ovarian, lung, thyroid cancer, and others. Doxorubicin exerts the chemotherapeutic function by intercalation in the DNA, disruption of the DNA repairing process mediated by topoisomerase-II and induction of cell damage by the production of ROS[12].
Gene delivery is a more recent therapeutic approach with potential for application in cancer treatment. MNPs conjugated with siRNA molecules are strong candidates for the development of multifunctional nanoplatforms that interfere with the protein translational processes in the cytoplasm and inhibit gene expression in tumor cells, see Figure 1. Due to the MNP properties, these nano-sized constructs display imaging and therapeutic applications, representing interesting theranostic agents[13].
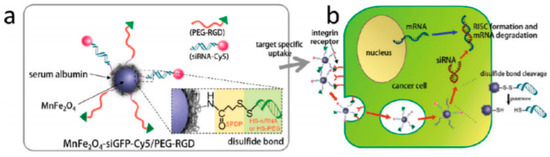
Figure 1. All-in-one nanoparticles of MnFe2O4siGFPCy5/PEG-RGD for theranostic purposes. (b) Schematic illustration of intracellular processes of MnFe2O4siGFPCy5/PEGRGD nanoparticles, from target-specific uptake to mRNA degradation. Reprinted with permission from[13].
To date, an increasing variety of magnetic nanoplatforms optimized for cancer theranostics have been developed and some relevant works are mentioned in Table 1.
Table 1. Magnetic nanoparticles tuned for dual imaging and therapeutic applications.
|
MNP System Description |
Characteristics |
Detection Methods |
Therapeutic Applications |
Tumor |
Reference |
|
Gold nanorod-capped magnetite core/mesoporous silica shell nanoparticles |
Mean diameter of 386.6 nm; homogenous size distribution; T2 relaxivity coefficient of 393.8 mM−1·s−1; Dox loading capacity of 30% w/w and positive therapy effect under 39–42 °C; no reported cytotoxicity <100 µg/mL; Absorption peak at 790 nm. |
MRI |
Doxorubicin chemotherapy; PTT |
- |
[14] |
|
Gold shell-core IONP |
Mean diameter: 100 nm; hydrodynamic size: 179 nm; T2 relaxivity coefficient of 76.2 mM−1·s−1 |
MRI; PAI |
PTT |
Breast |
[15] |
|
Multicore IONP with CuS shell |
Mean core diameter of 25.5 nm; hydrodynamic size: 156 nm; zeta potential: −14.1mV at pH 7; magnetization: 84 emu/g; |
MRI |
MHT; PTT; PDT |
- |
[10] |
|
cRGD-functionalized Doxorubicin-conjugated and 64Cu labelled SPION |
Mean core diameter:10 nm; mean hydrodynamic size of the MNP: 68 nm; T2 relaxivity coefficient of 101.9 mM−1·s−1; 64Cu T1/2:12.7 h; Dox-loading capacity of 5.8% w/w. |
PET; MRI |
Doxorubicin chemotherapy |
Glioblastoma |
[16] |
|
Indium-111 labeled Trastuzumab-Doxorubicin Conjugated, and APTES-PEG coated SPION |
Mean diameter: 16 nm; magnetization: 52 emu/g; radiolabel efficiency: 97.6%; trastuzumab conjugation capacity: 63.79%; |
SPECT; MRI |
Tumor suppression. Antibody and chemotherapeutic agents |
Breast |
[17] |
|
Manganese-doped iron oxide nanoparticles, coated with bovine serum albumin and functionalized with a cyclic Arg-Gly-Asp (cRGD) peptide and cy5 dye-labelled siRNA |
Mean core diameter:15 nm. |
MRI |
Inhibition of Green fluorescence protein by the siRNA moiety, and interference of receptor-mediated endocytosis via targeting tumor cells overexpressed αvβ3 integrin by RGD peptide. |
Breast |
|
|
Paclitaxel loaded, PEG modified liposome iron oxide MNP |
Core size of 7 nm; full nanoplatform size of 168.3 nm; PDI of 0.197; zeta potential of −10.5 mV; paclitaxel entrapment efficiency above 90%. |
MRI |
Paclitaxel |
Breast |
[20] |
|
Liposome, ADT loaded iron oxide MNP, encapsulated with PEG |
Core size of 7 nm; final size of 211 nm; PDI of 0.19; ADT loading capacity of 49.6%; T2* of 12.85 ms; |
MRI |
H2S |
Liver |
[21] |
|
Rituximab loaded liposome, iron oxide MNP, encapsulated with PEG |
Superparamagnetic NP-PVA core size average between 7–10 nm; narrow size distribution (PDI 0.1–0.3); 44.6% SPION-PVA encapsulation efficiency; zeta potential of −9.0 mV. |
MRI |
Rituximab |
Brain Lymphoma |
[22] |
Key: SPION—superparamagnetic iron oxide nanoparticle; MRI—Magnetic resonance imaging; PAI—Photoacoustic imaging; SERS—Surface Enhanced Raman Spectroscopy; APTES—aminopropyl triethoxysiliane; PEG—poly(ethylene glycol); cy5 dye—cyanine dye; cRGD—cyclic arginine-glycine-aspartate peptide; RGD—arginyl-glycyl-aspartic acid; ADT—hydrophobic anethole ditholethione; US—ultrasound; NIR—Near infrared.
Cancer nanotheranostic field is currently in expansion and the technological evolution requires continuous development of safer, more specific, sensitive and cost-effective strategies, in order to meet the required efficiency and efficacy for the MNPs’ performance[23].
This entry is adapted from the peer-reviewed paper 10.3390/ma13020266
References
- N. V. Srikanth Vallabani; Sanjay Singh; Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 1-23, 10.1007/s13205-018-1286-z.
- Palihawadana-Arachchige, M.; Naik, V.M.; Vaishnava, P.P.; Jena, B.P.; Naik, R. Nanostructured Materials—Fabrication to Application: Gd-Doped Superparamagnetic Magnetite Nanoparticles for Potential Cancer Theranostics. In School of Enviromental Sciences; Seehra, M.S., Ed.; InTech: Rijeka, Croatia, 2017; pp. 79–109. ISBN 978-953-51-3372-8.
- Sara Khademi; Saeed Sarkar; Sharmin Kharrazi; Seyed Mohammad Amini; Ali Shakeri-Zadeh; M. R. Ay; Hossein Ghadiri; Evaluation of size, morphology, concentration, and surface effect of gold nanoparticles on X-ray attenuation in computed tomography. Physica Medica 2018, 45, 127-133, 10.1016/j.ejmp.2017.12.001.
- Guillaume Thomas; Julien Boudon; Lionel Maurizi; Mathieu Moreau; Paul Walker; Isabelle Severin; Alexandra Oudot; Christine Goze; Sophie Poty; Jean-Marc Vrigneaud; et al. Innovative Magnetic Nanoparticles for PET/MRI Bimodal Imaging. ACS Omega 2019, 4, 2637-2648, 10.1021/acsomega.8b03283.
- Paul Beard; Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602-631, 10.1098/rsfs.2011.0028.
- Irina Belyanina; Olga S. Kolovskaya; Sergey Zamay; Ana Gargaun; Tatiana N. Zamay; Anna S. Kichkailo; Targeted Magnetic Nanotheranostics of Cancer. Molecules 2017, 22, 975, 10.3390/molecules22060975.
- Li, Y.; Wei, Q.; Ma, F.; Li, X.; Liu, F.; Zhou, M. Surface-enhanced Raman nanoparticles for tumor theranostics applications. Acta Pharm. Sin. B 2018, 8, 349–359.
- Kumar, G.V.; Rangarajan, N.; Sonia, B.; Deepika, P.; Rohman, N.; Narayana, C. Metal-coated magnetic nanoparticles for surface enhanced Raman scattering studies. Bull. Mater. Sci. 2011, 34, 207–216.
- Manuel Bañobre-López; Antonio Teijeiro; Jose Rivas; Magnetic nanoparticle-based hyperthermia for cancer treatment. Reports of Practical Oncology & Radiotherapy 2013, 18, 397-400, 10.1016/j.rpor.2013.09.011.
- Alberto Curcio; Amanda K. A. Silva; Sonia Cabana; Ana Espinosa; Benoit Baptiste; Nicolas Menguy; Claire Wilhelm; Ali Abou-Hassan; Iron Oxide Nanoflowers @ CuS Hybrids for Cancer Tri-Therapy: Interplay of Photothermal Therapy, Magnetic Hyperthermia and Photodynamic Therapy. Theranostics 2019, 9, 1288-1302, 10.7150/thno.30238.
- Oliviero L. Gobbo; Kristine Sjaastad; Marek W. Radomski; Yuri Volkov; Adriele Prina-Mello; Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249-1263, 10.7150/thno.11544.
- Caroline F. Thorn; Connie Oshiro; Sharon Marsh; Tina Hernandez-Boussard; Howard McLeod; Teri E. Klein; Russ B. Altman; Doxorubicin pathways. Pharmacogenetics and Genomics 2011, 21, 440-446, 10.1097/fpc.0b013e32833ffb56.
- Dongwon Yoo; Jae-Hyun Lee; Tae-Hyun Shin; Jinwoo Cheon; Theranostic Magnetic Nanoparticles. Accounts of Chemical Research 2011, 44, 863-874, 10.1021/ar200085c.
- Ming Ma; Hangrong Chen; Yu Chen; Xia Wang; Feng Chen; Xiangzhi Cui; J. L. Shi; Au capped magnetic core/mesoporous silica shell nanoparticles for combined photothermo-/chemo-therapy and multimodal imaging. Biomaterials 2012, 33, 989-998, 10.1016/j.biomaterials.2011.10.017.
- Jie Huang; Miao Guo; Hengte Ke; Cheng Zong; Bin Ren; Gang Liu; He Shen; Yufei Ma; Xiaoyong Wang; Hailu Zhang; et al. Rational Design and Synthesis of γFe2O3@Au Magnetic Gold Nanoflowers for Efficient Cancer Theranostics. Advanced Materials 2015, 27, 5049-5056, 10.1002/adma.201501942.
- Xiaoqiang Yang; Hao Hong; Jamison J. Grailer; Ian J. Rowland; Alireza Javadi; Samuel A. Hurley; Yuling Xiao; Yunan Yang; Yin Zhang; Robert J. Nickles; et al. cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials 2011, 32, 4151-4160, 10.1016/j.biomaterials.2011.02.006.
- Hamidreza Zolata; Fereydoun Abbasi-Davani; Hossein Afarideh; Synthesis, characterization and theranostic evaluation of Indium-111 labeled multifunctional superparamagnetic iron oxide nanoparticles. Nuclear Medicine and Biology 2015, 42, 164-170, 10.1016/j.nucmedbio.2014.09.007.
- Lee, J.H.; Lee, K.; Moon, S.H.; Lee, Y.; Park, T.G.; Cheon, J. All-in-One target-cell-specific magnetic nanoparticles for simultaneous molecular imaging and siRNA delivery. Angew. Chem. Int. Ed. 2009, 48, 4174–4179.
- Danhier, F.; Breton, A.L.; Préat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973.
- Catarina Oliveira Silva; Jacinta Oliveira Pinho; Joana Margarida Lopes; António J. Almeida; Maria Manuela Gaspar; Catarina Reis; Current Trends in Cancer Nanotheranostics: Metallic, Polymeric, and Lipid-Based Systems. Pharmaceutics 2019, 11, 22, 10.3390/pharmaceutics11010022.
- Yang Liu; Fang Yang; Chuxiao Yuan; Mingxi Li; Tuantuan Wang; Bo Chen; Juan Jin; Peng Zhao; Jiayi Tong; Shouhua Luo; et al. Magnetic Nanoliposomes as in Situ Microbubble Bombers for Multimodality Image-Guided Cancer Theranostics. ACS Nano 2017, 11, 1509-1519, 10.1021/acsnano.6b06815.
- S. Saesoo; S. Sathornsumetee; P. Anekwiang; C. Treetidnipa; Peti Thuwajit; S. Bunthot; W. Maneeprakorn; Lionel Maurizi; H. Hofmann; Ruktanonchai Uracha Rungsardthong; et al. Characterization of liposome-containing SPIONs conjugated with anti-CD20 developed as a novel theranostic agent for central nervous system lymphoma. Colloids and Surfaces B: Biointerfaces 2018, 161, 497-507, 10.1016/j.colsurfb.2017.11.003.
- Hong Yu Yang; Yi Li; Doo Sung Lee; Multifunctional and Stimuli-Responsive Magnetic Nanoparticle-Based Delivery Systems for Biomedical Applications. Advanced Therapeutics 2018, 1, 1800011, 10.1002/adtp.201800011.
