Biomimetic Hybrid Systems for Tissue Engineering: New Materials and Fabrication Techniques
- tissue engineering
- biomimetic proteins
- biomimetic DNA
- biomimetic sca olds
- melt electrospinning
- hybrid technologies
- hard and soft tissues regeneration
- stem cells
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition:
Tissue engineering approaches appear nowadays highly promising for the regeneration of injured/diseased tissues. Biomimetic scaffolds are continuously been developed to act as structural support for cell growth and proliferation as well as for the delivery of cells able to be differentiated, and also of bioactive molecules like growth factors and even signaling cues. The current research concerns materials employed to develop biological scaffolds with improved features as well as complex preparation techniques. In this work, hybrid systems based on natural polymers are discussed and the efforts focused to provide new polymers able to mimic proteins and DNA are extensively explained.
1. Introduction
Nowadays, great efforts are focused on the design of biodegradable scaffolds for applications in the biomedical field such as tissue engineering and organ regeneration. Natural and synthetic polymers and even their blends have been tested, but an ideal system has not yet been developed due to the multiple requirements that must be met. These involve chemical, physical, and biological properties. For example, the rate of degradation must be adjustable and the corresponding degradation products must not be toxic, the scaffolds produced from the selected material must retain their mechanical integrity before regeneration, the material should guarantee immunogenicity, and even should have capacity to tune cell adhesion, proliferation, and differentiation. Scaffolds with multifunctional properties (e.g., mechanical performance, biocompatibility, surface adhesion) are consequently often required, being the best options focused on the use of hybrid systems that combine different materials [1,2].
Incorporation of stem cells in the scaffolds appears fundamental to enhance healing processes. These cells can be differentiated under the action of an opportune stimuli while homeostasis in the healthy tissue could be kept. Performance of designed scaffolds can be improved by the additional incorporation of active agents to stimulate tissue regeneration (e.g., growth factors) and even to avoid infection risk and biofilm formation (e.g., bactericide drugs) [3,4].
Usually, scaffolds developed for tissue engineering try to mimic the natural extracellular matrix (ECM). This is constituted by different entrapped proteins (e.g., collagen, laminin, or fibronectin) that act as cell binding ligands. Specifically, integrin-recognizing peptide sequences are fundamental since they provide an inherent cell adhesivity [1,5] between the cellular cytoskeletons and the ECM microenvironment.
A good control of porosity, interconnectivity between pores, and even the size of pores is fundamental to provide appropriate 3D-scaffolds for the different kinds of tissues (e.g., hard or soft). Therefore, great efforts are focused to the development of new processing technologies able to achieve the indicated goals. In fact, macro-, micro-, and nanoarchitecture of 3D scaffolds have a primordial relevance to replicate the structural complexity of living tissues. Hybrid systems considering both material mixtures and a combination of fabrication processes are nowadays fundamental to mimic natural tissues by providing multiphasic or multimaterial structures [6,7,8].
2. Biomimetic Hybrid Systems Based on Natural Polymers for Tissue Engineering
Tissue engineering applications require the use of complex scaffolds where an accurate selection of materials is essential. In addition, it is advantageous that these materials could accommodate growth factors and cells, while providing cues to guide cell adhesion and proliferation [9,10].
Hydrogel scaffolds appear ideal matrices to culture cells and produce in vitro tissues [11]. Probably, polysaccharides (Figure 1) constitute the most interesting family of polymers due to their high hydrophilicity, their origin linked to the living tissues, their biodegradable and biocompatible properties, their ability to render hydrogels with similar properties to ECM [12], and their capacity to display bio-responsive functions [13]. Nevertheless, chemical modifications appear necessary to facilitate the attachment of cells, which may be a problematic feature since a complete removal of employed toxic agents is essential for subsequent biomedical applications [14]. Direct blending of polysaccharides is a recent strategy that is applied to improve cellular adhesion and proliferation as recently reviewed by Ng et al. [9].
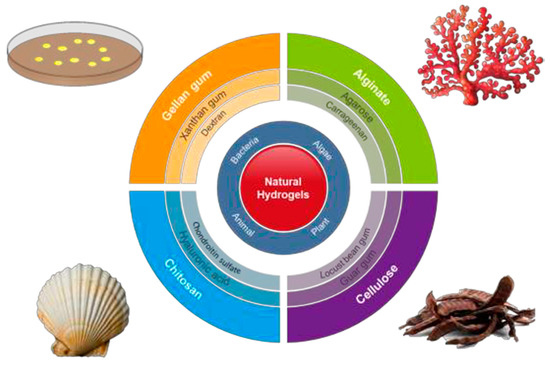
Figure 1. Natural polysaccharides able to produce hydrogels and respective sources. Reproduced with permission from [9].
Cellulose and chitosan are between the most employed hydrogels for biomedical applications. In fact, they are the most abundant natural polymers in earth. Cellulose appears an ideal matrix for tissue engineering applications [15], alone or even blended [16] with other polymers like chitosan. Hydrogels can be prepared by crosslinking of aqueous cellulose esters [17], a procedure that avoids the limitations caused by insolubility of natural cellulose in aqueous media. Nanocellulose (i.e., cellulose nanocrystals [18], CNCs, cellulose nanofibers [19], NFCs, and bacterial cellulose [20], BC) can also be incorporated into polymer matrices to improve mechanical properties. For example, the interpenetrating network of gelatine and alginate reinforced with CNCs can improve the performance of natural cartilage, favor cell adhesion, and protect cells from immune rejection [21]. A second interesting example about the use of nanocellulose corresponds to the development of BC membranes coated with alginate and collagen in each side [22].
Deacetylation of chitin leads to chitosan, which has a great structural stability, capability to absorb water, and a cationic character that favors the formation of gel particles through electrostatic interactions [23]. Chitosan is mostly employed as injectable hydrogels, crosslinking agents like genipin [24] and even hydroxyapatite particles being added to improve osteogenic properties and enhance cell adhesion and proliferation [25].
Collagen is the most relevant structural protein in the human body [26] and is the major component of the native ECM. Therefore, great efforts have been focused on preparing collagen-based scaffolds to mimic native environment [27]. Solution processing (e.g., electrospinning) of collagen has some restrictions since solvents used to denaturalize the highly insoluble triple helix structure (e.g., 1,1,1,3,3,3-hexafluoroisopropanol) should be avoided due to their toxicity [28]. To this end, co-electrospinning has been proposed using a biodegradable synthetic polymer able to provide elasticity (e.g., the copolymer of l-lactide and ε-caprolactone) and a mixture of collagen and a water-soluble sacrificing polymer (e.g., PVP) that acts as a transporter polymer for an easy spinning of collagen [29].
Different scaffolds have successfully been developed to mimic the 3D organization of interstitial ECM, but scarce results have been reported concerning the reproducibility of the 2D ECM basement membrane (BM) [30,31]. These membranes establish functional polarization of epithelial and endothelial cell layers throughout the entire body [32,33] and appear fundamental for artificial organ technologies [30,34]. Furthermore, BMs assure tissue compartmentalization and may prevent the spreading of cancer cells [35]. Basically, BMs are based on the assembly of two different proteins: type IV collagen (Col IV) and laminin (LM), which provides unique mechanical properties for an effective protection of tissues from external stresses [36]. Artificial matrices mimicking BMs have been prepared by co-assembling polylaminine and Col CV under acidic conditions. A layered morphology (thickness around 15 m) was derived, its great capacity to support keratinocytes and form a cell layer close to the architecture of natural epidermis being demonstrated [31].
Gelatine, as a soluble denaturalized collagen, is widely employed for tissue regeneration due its relevant role in cellular metabolism and morphogenesis. Moreover, gelatine is interesting for giving rise to thermosensitive scaffolds taking into advantage its capability to form solid gels at low temperature through intermolecular hydrogen bonding interactions [37], which can be disturbed at increasing temperatures to form liquid gels.
Mixtures of gelatine with other natural polymers like agar and alginate have also been evaluated. Thus, agar–gelatine mixtures have been found appropriate to produce hydrogel scaffolds with a uniform internal pore structure [38], and gelatine/alginate mixtures were found suitable for manufacturing biological scaffolds by 3D bioprinting [39]. Natural gelatine has also been blended with synthetic polymers such as nylon 6 and polyurethane to render suitable scaffolds for bone tissue engineering [40]. New scaffolds facilitated apatite-like mineral deposition, and promoted osteoblast cell attachment, migration, and proliferation [41]. Hybrid nanofibrous scaffolds have also been prepared by layering a poly(3HB-co-4HB) copolymer and gelatine [42]. The trilayered system with gelatine in the middle showed a good water-resistant ability and high proliferative activity of mouse fibroblasts. Hydrogels based on gelatine and methacrylamide have been found appropriate to simulate liver tissue, a hepatocyte cell survival rate of 97% being found [43]. It can be deduced that hybrid composite materials with natural and synthetic polymers are gaining relevance to develop biomimetic scaffolds [44,45,46].
Fibrin, keratin, alginate, and hyaluronic acid are other relevant natural polymers that are gaining relevance for the development of biomimetic scaffolds. Fibrin is a structural protein employed to prepare bioprinted scaffolds [47]. Specifically, scaffolds with embedded neurons have been, for example, prepared by a layer-by-layer printing process These materials can attain modulus and tensile strength of 2.9 and 1.7 MPa, respectively [48]. The fast gelation rate of fibrin can be combined also with the high biocompatibility of collagen to render scaffolds with fast wound closure and high vascularization [49].
Keratin is a structural fibrous protein that forms part of epidermal structures (e.g., nails, wool, hair, and feathers) and provides toughness and resilience depending on the number of sulfur cross-links that are established through their cysteine units [50,51]. Keratin can be extracted from natural materials by different processes such as the use of ionic liquids [52], microwave irradiation [53], alkaline treatment [54], and reduction of disulphide linkage [55]. Capability of keratin to self-assemble and support cellular proliferation enhance its use in tissue regeneration applications [50,56]. Between them, the reconstruction or regeneration of ocular surface [57], urinary track [58], and nerves [59] are significant. The relatively poor mechanical properties and brittle structure of keratin justified its blending with different synthetic (e.g., poly(ε-caprolactone (PCL), polylactide (PLA), polyvinyl alcohol, and polyethylene oxide) and natural (e.g., chitosan, gelatine, silk, fibrin, and hyaluronic acid) polymers [51].
Sodium alginate is a natural polysaccharide widely employed in tissue engineering despite some limitations like low mechanical integrity to form suitable fibers and too high biodegradation rate to be used as a supporting matrix. Nevertheless, this polysaccharide has several advantages for cartilage tissue engineering as recently reviewed [60].
Hyaluronic acid (HA) is a glycosaminoglycan that can give rise to fibrillar structures with good mechanical performance and a regulatory function [61]. HA has interest in regeneration processes [62] and consequently is considered in tissue engineering applications. Fabrication of HA scaffolds with high porosity (i.e., 70–95%) and swelling ratio (i.e., 2000–6000%) has been reported. These materials display soft-tissue mimetic mechanical properties (≈0.5–1.5 kPa), accelerated tissue formation, neovascularization, and reepithelialization in vivo [63].
Interest towards peptide hydrogels is emerging despite their higher cost with respect to typical polysaccharide-based materials. Efforts are logically focused to mimic the natural fibrillar proteins of ECM by means of synthetic peptides able to self-assemble. Different morphologies are derived depending on the peptide sequence. Basically, strategies to favor self-assembly are based on (a) an alternate disposition of hydrophilic and hydrophobic residues (e.g., Arg-Ala-Asp-Ala characteristic of the named RADA peptides) [64]; (b) the use of complementary peptides (i.e., peptides having opposite electric charges) [65]; (c) peptide amphiphiles [66]; (d) cyclic peptides [67]; (e) functionalized peptides [68] (Figure 2). High cost, non-renewability, complex purification processes, and demanding storage conditions are some drawbacks that limited the use of protein-based hydrogels despite their inherent cell adhesivity [69,70].
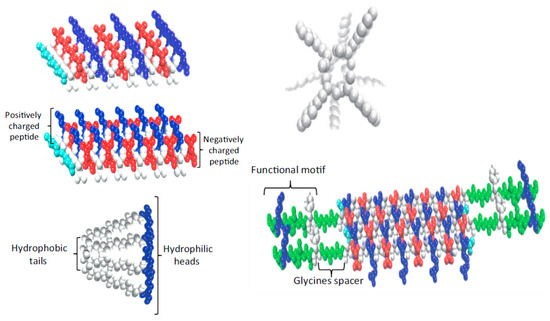
Figure 2. Strategies developed to favor peptide self-assembly: alternate disposition of hydrophilic and hydrophobic residues, complementary co-assembling peptides, peptide amphiphiles, cyclic peptides, and functionalized peptides. Reproduced with permission from [70].
Electrostimulation (ES) has beneficial effects on tissue regeneration (i.e., muscle, bone, skin, nerve, tendons, and ligaments) since it enhances cell proliferation, ECM synthesis, cytokines production, and vasculature development [71]. The development of scaffolds based on electroactive polymers is highly interesting to facilitate the successful application of ES. Conductive polymers (i.e., based on electronic conductivity enabled through oxidized pi-bond conjugation states) are interesting but have poor properties (in general are brittle and non-degradable thermosets with a conductivity that decreases progressively as a reduced state is attained). Usually, composite systems based on conductive polymers like polypyrrole, polyaniline, and polythiophene derivatives, and natural polymers like chitosan, cellulose, and alginate have been selected. Alternatively, the incorporation of ionic functional groups (e.g., carboxylic, sulfonic, and amine) to a polymer can generate ionic conductive materials without modifying significantly biocompatibility and degradation profiles [72].
Incorporation of carbon nanotubes promote cell proliferation and facilitate nerve regeneration. Conductive hydrogels have been prepared from N-isopropylacrylamide (NIPAM), laponite, and CNTs [73], also being remarkable the derived improvement of mechanical properties. This characteristic was considered to prepare hybrid hydrogels constituted by gelatine, sodium alginate, and CNTs to be employed as bioink to print hollow tubular scaffolds. These led to blood vessels after colonization by epidermal fibroblasts. Doping with CNTs was essential to enhance mechanical properties while no cytotoxicity was found [39].
3. Conclusions
Accurate selection of materials is essential to develop appropriate scaffolds for tissue engineering applications. These materials should allow incorporating growth factors and host cells while providing cues to guide cell adhesion and proliferation. Natural polymers like fibrin, keratin, chitosan, and the different forms of collagen have been successfully employed. Probably, hydrogel scaffolds appear the most ideal matrices to culture cells and produce in vitro tissues, polysaccharides being highly appropriate due to their hydrophilicity, biodegradability, and biocompatibility. Bio-responsive functions and similar properties to ECM can be attained although chemical modifications are still necessary to facilitate the cell attachment.
Despite their higher cost, the development of peptide hydrogels is gaining attention. Efforts are nowadays also focused on the incorporation of electroactive polymers and conductive nanoparticles due to the beneficial effect of electrostimulation on tissue regeneration. The use of elastomeric materials has also a high interest for tissue engineering applications as a consequence of their chemical inertness and their suitable mechanical properties.
Among the artificial degradable polymers designed for biomedical applications one of the most promising are polymers mimicking naturally occurring polymers—proteins and nucleic acids. These biomimetic systems have numerous advantages over the natural polymers such as wider range of material properties, higher safety and higher reproducibility, excellent compatibility with tissues, lower price, to name a few. This makes the biomimetics highly promising for wide biomedical applications as resorbable surgical materials and drug delivery systems.
Considering the design of polymer for desired biomimetic applications, the selection of processing and fabrication methods is primordial. Among different techniques, electrospinning and more specifically eco-friendly melt electrospinning and writing attract more attention to provide a nontoxic environment for biomimetic applications. Further improvements are possible combining two or more techniques, rendering products with suitable physicochemical, cell proliferation, and mechanical properties. For example, electrospinning of hybrid systems, coelectrospinning and coaxial electrospinning, two step hydrogel production, combination of melt electrowriting and electrospinning have been demonstrated as effective ways for fabricating new and modified scaffolds for tissue engineering.
Synthetic and natural polymers are being continuously tested and modified to design biomimetic scaffolds for a wide range of tissue engineering applications such as healing of skin and wounds, reconstruction of bone, cartilages and muscles, reparation of the nervous system, regeneration of vasculature, and even drug delivery and gene therapy. Recent designs are focused to create multifunctional structures able to provide complex biological functions. New therapies can potentially regenerate many types of tissues and organs, but some limitations like acute cell death, uncontrolled differentiation, and low functional engraftment yields still need attention. Efforts should also be focused to improve the understanding of interactions between cells and ECM, to tune the structure of scaffolds in order to simulate more accurately the ECM structures and topologies, and to insist on the preparation of stimuli responsive hybrid biomaterials.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics5040049
