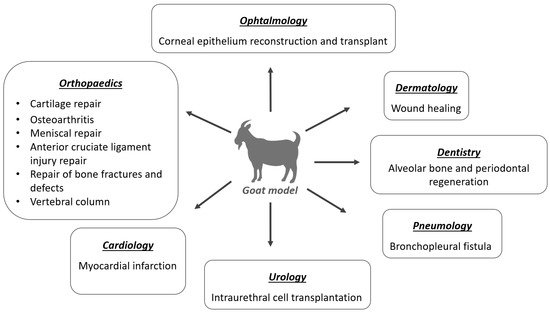
3. Application of MSCs in the Goat Model
3.1. Orthopedics
MSCs are an alternative source of cells for cartilage, muscle, tendon, and bone regeneration, as they are easily accessible and can be harvested from different tissues, having a great capacity to proliferate and differentiate into other sorts of cells in the body, such as osteoblasts and chondroblasts [
37,
46,
47]. Caprine models have been used in preclinical and translational research studies to assess new approaches, resorting to MSCs for cartilage tissue engineering aiming for regenerative joint resurfacing, osteoarthritis treatments, bone fractures and defects, menisci and anterior cruciate ligament injury repair studies. Additionally, several studies have been performed on the goat at the spine level, namely for research on new treatments for intervertebral disc (IVD) disease.
3.2. Dermatology
Chronic cutaneous wounds and ulcers represent a therapeutic challenge, due to the difficulty of clinical management, high recumbence rate, and scar formation, both in human and veterinary medicine. Its incidence has increased due to population aging, diabetes, obesity, and concomitant diseases [
2].
MSCs present an important role in all phases of tissue repair: inflammation, proliferation, and remodeling [
2,
3]. These cells also promote angiogenesis and show evidence of antimicrobial properties. A failure in the angiogenesis process during wound healing can induce the development of chronic wounds [
2,
3]. MSCs have already been shown to promote wound healing through a paracrine molecular cascade in goat models [
128,
129]. Furthermore, MSCs may have direct involvement in various stages of the wound healing process that need to be further explored [
129].
Although goats have not generally been used as research models for cutaneous wound healing, they are a good choice as they have a mild temperament and a good anatomical skin surface available for creating lesion models of different shapes and sizes [
16].
Studies with the goat model for wound healing have used different sources of MSCs with excellent results. Pratheesh et al. (2017) showed evidence of better wound healing with MSCs from the amniotic fluid origin than caprine BMSCs, by revealing greater epithelialization, neovascularization, and collagen development in the histomorphometric analysis [
130]. Azari et al. (2011) also showed the re-epithelization capacity of transplanted Wharton’s jelly MSCs from caprine umbilical cords, revealing complete re-epithelization of cutaneous wounds in 7 days [
131].
In addition, MSCs showed great capacity for wound regeneration and reduced healing time and plasticity, as they are capable of converting into cells of different tissues. A study carried out by Yang et al. (2007) with goats confirmed that epidermal adult stem cells can differentiate into different functional cells in vivo or in vitro, demonstrating the plasticity of stem cells [
132].
3.3. Ophthalmology
In recent years, ophthalmologists have placed a great focus on stem cells to treat various traumatic and degenerative disorders due to their unique biologic properties [
133]. The cornea is a protective barrier and is formed by three layers with different germinal origins: the epithelium (originated from superficial ectoderm) and the stroma and endothelium (originated from neural crest cells) [
134]. Experimental studies have proved that there is a variety of stem cells present in each of these layers [
134,
135]. For example, limbal stem cells (residing in the limbus) maintain epithelial homeostasis and regenerate the cornea, with epithelial cell deficiency being the leading cause of blindness worldwide [
134].
Stem cells have high potential in the treatment of eye diseases characterized by permanent cell loss, such as glaucoma, age-related macular degeneration, photoreceptor cell degeneration, hereditary retinopathy, and mechanical and ischemic retinal injuries [
134,
136,
137]. The eyes of small ruminants are anatomically different from human eyes [
16,
138], however the resemblance in structure, size with, some properties and parameters to the human eye made possible to use these models successfully. Goats have been used mostly to study corneal epithelium reconstruction and transplant.
Studies have shown that epidermal adult stem cells (EpiASCs) from goat ear skin can be used to successfully repair damaged cornea with total limbal stem cells (LSCs) deficiency [
139,
140]. Moreover, these results demonstrated that EpiASCs can be induced to differentiate into corneal epithelial cell types in vivo in a corneal microenvironment, and had the skill to trigger corneal genetic programs [
139].
In a study carried out by Mi et al. (2008), cryopreserved limbal corneal stem cells were applied in goats with damaged cornea with excellent results. The therapeutic effect of transplantation may be associated with the inhibition of inflammation-related angiogenesis after transplantation of cryopreserved LSCs [
141].
3.4. Dentistry
There are only a few published studies with goats for stem cell therapy in dentistry, probably due to the anatomical differences between human and goat dentition. Because it is a ruminant species, it only has incisors in the mandible, being absent in the upper jaw. It has no canines and only premolars and molars, the latter fulfills the function of rumination of plant foods. Stem cell research in dentistry aims at the regeneration of damaged tissues such as periodontal tissues, dentin, pulp, and resorbed roots and the repair of endodontic iatrogenic perforations [
142]. It is mainly used for periodontal regeneration and in association with biomaterials to optimize tooth regeneration in the goat model [
143,
144]. Undifferentiated MSCs are able to differentiate, providing the three critical tissues essential for periodontal tissue regeneration: cementum, bone, and periodontal ligaments, making stem cells a new approach for periodontal tissue regeneration [
145]. The association of MSCs and fibrin glue using particulate mineralized bone has also shown promising results for vertical bone augmentation in animal models [
146].
There is a growing need to use dental implants and improve their function to enhance normal dental physiology and proprioception [
142]. An osseointegrated implant can closely resemble a natural tooth. Nevertheless, the absence of periodontal ligament and connective tissue results in important differences in implant adaptation to occlusal forces [
145]. Several studies for tooth regeneration with scaffolds based on biomaterials and stem cells have shown very positive effects on regeneration [
143,
144,
145,
147]. Dense collagen gel scaffolds seeded on MSCs and nanostructured titanium surfaces have increased interest in bone regeneration due to the good osteointegration effect [
148,
149].
The placement of implants can be problematic, as, after a tooth loss, anatomical pneumatization of the maxillary sinuses can occur, as well as atrophy of the alveolar ridge, limiting the bone volume available to the implant placement. Maxillary sinus floor elevation is one of the preferred surgery options to solve this problem, where bone graft material is placed in the maxillary sinus to provide adequate support to the implants [
143]. Zou et al. (2012) associated this grafting material with BMSCs and calcium phosphate cementum, promoting earlier bone formation and mineralization, and maintaining the height and volume of the augmented maxillary sinus in a goat model [
143].
Bangun et al. (2021) proved that MSCs can improve earlier bone repopulation and complete faster bone regeneration in tissue-engineered bone grafts, supported by the paracrine activity of the resident stem cells [
144].
It is also known that TGF-β1 plays an important role during tooth formation, this GDF can directly induce the differentiation of odontoblast-like cells, and positively regulate the secretion of matrix components in the dentin-pulp complex, being a potential therapy to induce tissue formation after dental pulp capping treatments [
150].
3.5. Pneumology
Stem cells have been a promising therapy for asthma non-responsive to conventional therapy [
1,
42], as a potential treatment for destructive lung diseases including chronic obstructive pulmonary disease [
151], and as a treatment for bronchopleural fistula (BPF), due to its plasticity and ability to differentiate into different cells [
152].
Goats have been successfully used in a bronchopleural fistula model. In cases of lung cancer with limited disease, the most effective method of controlling the primary tumor is surgical resection, as it offers the best chance of cure. Pulmonary resection can lead to the development of a pathological connection between the airway (bronchus) and the pleural space, known as a post-resection BPF [
152]. This research proved that bronchoscopic-guided transplantation of BMSCs successfully closes bronchopleural fistula by extraluminal fibroblast proliferation and collagenous matrix development [
152].
3.6. Cardiology
The goat seems to be the ideal model for cardiovascular diseases, due to its anatomical dimensions and physiological similarities to the human heart. Additionally, parameters such as heart rate, coronary architecture, and capillary density are more similar to those of man [
9,
14].
One of the main causes of morbidity and mortality in cardiovascular diseases is myocardial infarction, characterized by the ischemic lesions of cardiac muscle tissue due to an occlusion of one of the coronary arteries or one of its branches by a thrombus [
17]. This disease is increasing and gaining more importance due to the general aging of the population and the change in lifestyle [
17,
18].
The goat has been studied for the treatment of myocardial infarction through in vitro and in vivo studies. So far, several different sources of adult stem cells have been identified as a healing approach for infarcted myocardium. The most promising stem cells that have so far shown the best results in these studies are BMSCs. A study carried out by Liao et al. (2006) uses BMSCs enriched by small intestinal submucosal (SIS) films to treat myocardial infarction in goat models, the MSCs-SIS film was implanted and sutured in the infarct area. The obtained results revealed that this therapy can prevent ventricular chamber dilatation and can improve myocardium contractility, cardiac function, and collateral perfusion [
18].
Another promising source of stem cells is glandular stem cells that can be easily extracted from exocrine glands, such as the salivary glands or the pancreas [
17,
153,
154]. Maass et al. (2009) showed that glandular stem cells obtained from the submandibular gland can spontaneously differentiate into cardiac-like mesodermal cells in vitro. These results suggest that implanting these cells directly into infarcted myocardium can improve heart regeneration [
17].
3.7. Urology
Cell-based therapy is emerging as a great alternative in the treatment of stress urinary incontinence. Goats are also an ideal model to study this subject, as the female caprine urethra has similar parameters to those reported in humans, by measuring the urethral pressure profile, making them a suitable experimental animal for testing intraurethral cell transplantation [
155].
Burdzinska et al. (2017) demonstrated that caprine muscle-derived cells (MDCs) and MSCs can be expanded in vitro and applied for intraurethral injections [
155]. In 2018, Burdzinska proved that both MSCs and MDCs collaborated in the formation of striated muscle when they were transplanted directly into the external urethral sphincter. This study suggests that MDC-MSC co-transplantation improves urethral closure better than if transplantation of each cell population is performed alone [
156].