Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Varias enfermedades virales suelen afectar a la cavidad bucal; por ejemplo, la infección por el virus de la inmunodeficiencia humana (VIH) puede presentarse inicialmente con lesiones orales, la infección por el virus del papiloma humano (VPH) a menudo aumenta el riesgo de desarrollar carcinoma oral de células escamosas, y se ha documentado daño oral durante las infecciones por el virus de la hepatitis B y C.
- COVID-19
- cavity buccal
- oral lesion
1. Introducción
La enfermedad por coronavirus 2019 (COVID-19) es la patología causada por el síndrome respiratorio agudo severo coronavirus 2 (SARS-CoV-2), que es un virus que contiene en su material genético una sola hebra de ARN [ 1 ] . Algunos de los signos y síntomas clínicos más comunes de COVID-19 son fiebre, dolor de garganta, dolor de cabeza, dificultad para respirar, tos seca, dolor de estómago, vómitos y, a veces, diarrea [ 2 ] . El receptor 2 de la enzima convertidora de angiotensina (ACE2) es uno de los principales receptores conocidos del SARS-CoV-2 para ingresar a las células de los pulmones, el hígado, los riñones, el sistema gastrointestinal e incluso en el endotelio de los vasos papilares dérmicos y en el epitelio. superficies de las glándulas sudoríparas [ 3 ]. Se han descrito diversas manifestaciones cutáneas en pacientes con COVID-19, que incluyen pseudosabañones, lesiones variceliformes, lesiones similares al eritema multiforme, forma de urticaria, maculopapular, púrpura y petequias, moteado y lesiones similares a la livedo reticularis [ 4 ] [ 5 ] . En la cavidad oral, la ACE2 se expresa en la mucosa oral, especialmente y en mayor cantidad en la superficie lingual y en las glándulas productoras de saliva en relación con la mucosa de la boca o el paladar [ 6 ] . La disgeusia es el primer síntoma oral reconocido de COVID-19 informado en el 38 % de los pacientes, especialmente en norteamericanos y europeos y en pacientes con enfermedad de gravedad leve a moderada [ 4 ]. Desde que se describieron las primeras manifestaciones bucales asociadas al COVID-19, se han publicado varios reportes describiendo una amplia variedad de lesiones, donde la manifestación lesional bucal más frecuente es la ulceración [ 7 ] , además de placas blancas, petequias, lengua geográfica, máculas , nódulos, angina ampollosa, enfermedad periodontal necrosante, ampollas y lesiones similares a eritema multiforme [ 8 ] [ 9 ]. Se ha informado que la pérdida del gusto y/u olfato persiste hasta por 14 días y progresa más rápidamente en pacientes mayores; la recuperación de las lesiones bucales ocurre al mismo tiempo que los pacientes se recuperan de COVID-19, lo que representa una asociación entre las manifestaciones de todas las lesiones clínicas que aparecen en la boca y la infección por SARS-CoV-2 de los pacientes [ 10 ] .
Dado que las manifestaciones clínicas de COVID-19 más allá del daño pulmonar causado por la inflamación aún no se comprenden adecuadamente, las condiciones de salud oral asociadas con COVID-19 continúan estudiándose para obtener una mejor apreciación de las manifestaciones orales. La crisis que el mundo sigue enfrentando por el COVID-19 ha resaltado la importancia de comprender las condiciones implícitas que conducen a los resultados relacionados con el COVID-19, principalmente la mortalidad, así como la positividad y la gravedad [ 11 ] [ 12 ] [ 13 ] [ 14 ] .
2. La cavidad oral y su papel en la inmunidad
The oral cavity has three major host defenses against microbial invasion: the oral mucosa, nonspecific (innate) immunity, and adaptive (acquired) immunity. The oral mucosa consists of a layer of interconnected epithelial cells containing mainly keratinocytes resting on a basal membrane and provides a physical barrier that protects the underlying tissues from microorganisms and environmental threats in the oral cavity [15][16][17]. Cells of the immune system and oral keratinocytes in the lamina propria of the oral mucosa detect some pathogen-associated molecular patterns that have been conserved by evolution in specific classes of microorganisms [18][19]. Pattern recognition receptors distinguish between different molecular structures of microorganisms and thus prevent the generation of immunoinflammatory responses against these microorganisms [20][21]. Pattern recognition receptor families have been described in some reports, in which have been included for example the toll-like receptor family (TLR-1 to TLR-10) and the C-type lectin receptor family (Dectin-1, Dectin-2, dendritic cell specific intercellular adhesion molecule 3-grabbing nonintegrin) [22]. The initiation and determination of the type of specific adaptive immune responses induced by pattern recognition receptors also dictate the magnitude and duration of the responses and whether or not memory T cells are activated [20]. The specificity, type, and sensitivity of pattern recognition receptor-mediated adaptive immune responses are determined by the nature of the infectious agent, for example, if they are viruses, bacteria, fungi, and/or protozoan [23]. By the microenvironment characteristics, the type of cells expressing each family of pattern recognition receptors, the anatomical site where these cells are located, and the combination of interactions that occur between these factors [24]. Warning signals generated by some tissue-damaging factors, including hypoxia, radiation, and trauma to some extent affect the speed and magnitude of the immune response [25].
3. Oral Cavity during Viral and Bacterial Infections
Several viral diseases usually affect the oral cavity; for example, human immunodeficiency virus (HIV) infection may initially present with oral lesions, human papillomavirus (HPV) infection often increases the risk of developing oral squamous cell carcinoma, and oral damage has been documented during hepatitis B and C virus infections [26][27][28][29].
There is a combination of proteins with complementary dynamics in virus and/or bacterial infection in the oral cavity. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has been shown to be significantly stimulated in response to infections that are primarily viral in origin [30]. Interferon gamma-induced protein-10 (IP-10) levels are generally slightly elevated in patients with bacterial infections and highly elevated in patients with viral infections. It is also known that C-reactive protein (CRP) levels are commonly found to be increased in patients who have developed infections of bacterial origin and that CRP levels are less elevated in patients who have developed infections caused by viruses (Figure 1) [31].
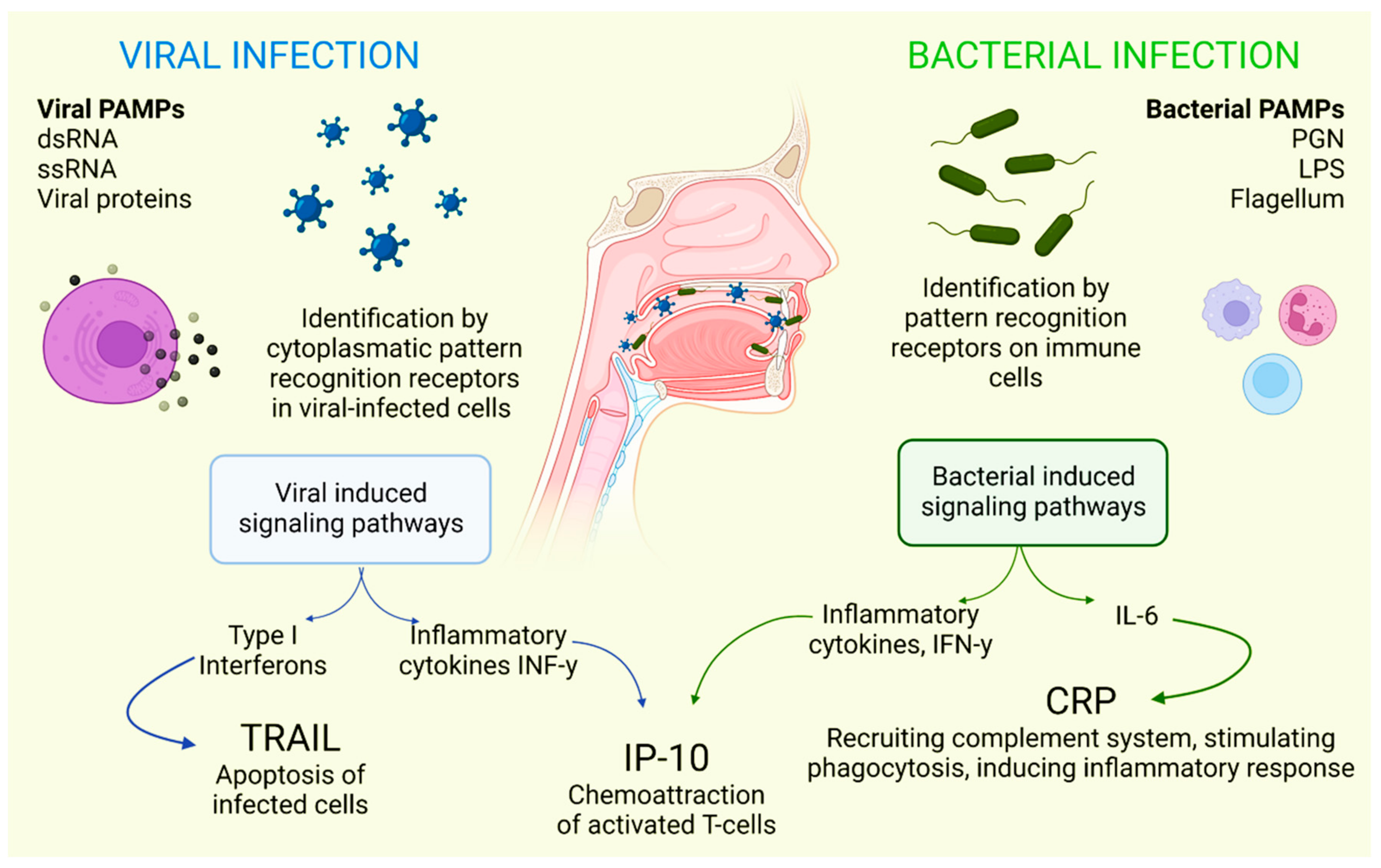
Figure 1. Selective host response against bacterial and viral infections. Different molecules and signaling pathways are dynamically involved in complementing the response to virus and bacterial infections (including CRP, IP-10 and TRAIL). IL-6: interleukin-6; LPS: lipopolysaccharide; PAMPs: pathogen-associated molecular patterns; PGN: peptidoglycan; ssRNA: single-stranded RNA; dsRNA: double-stranded RNA. Adapted with permission from Oved, K. et al. (2015) [32], which was distributed under the terms of the Creative Commons Attribution License https://creativecommons.org/licenses/by/4.0/; accessed date 28 November 2021.
Some drugs used in the treatment of viral infections can also contribute to damage to the oral cavity. High doses of corticosteroids may trigger fungal infections such as oral candidiasis; antiviral drugs can cause dry mouth, aphthous ulcers, and stomatitis; and the use of antiviral drugs can cause dry mouth [33]. Additionally, many patients have been prescribed antibiotics that are effective against Gram-negative and Gram-positive bacteria, which usually has a direct impact on the homeostasis of the mouth and all microorganisms found in this cavity [34].
Oral Cavity and SARS-CoV-2 Infection
As mentioned before, SARS-CoV-2 is an RNA-positive virus with an icosahedral morphology that possesses S proteins that are the binding site for ACE2 in humans [1], which, in addition to the lungs, pancreas, adipose tissue, liver, or kidney, this receptor is also expressed in salivary glands [35]. The oral cavity is a gateway for many pathogens, and SARS-CoV-2 is not the exception. This virus is detected in the saliva of all COVID-19 patients even with more sensitivity than that of nasopharyngeal testing [36].
When the ACE2 protein of the host cell and the S protein of SARS-CoV-2 bind, an interaction occurs that allows the coronavirus to use the machinery of these host cells to replicate and subsequently destroy these same cells, triggering the oral symptoms and signs [37]. In addition to this mechanism, which explains the cause of several manifestations of oral lesions caused by COVID-19, it is also possible that these lesions are the result of opportunistic infections that facilitate immune system alterations and may also be facilitated by possible systemic damage and adverse effects that can be triggered by treatment [38]. Figure 2 represents the location of ACE2 in different tissues and structures of the oral cavity, as well as those interacting with SARS-CoV-2.
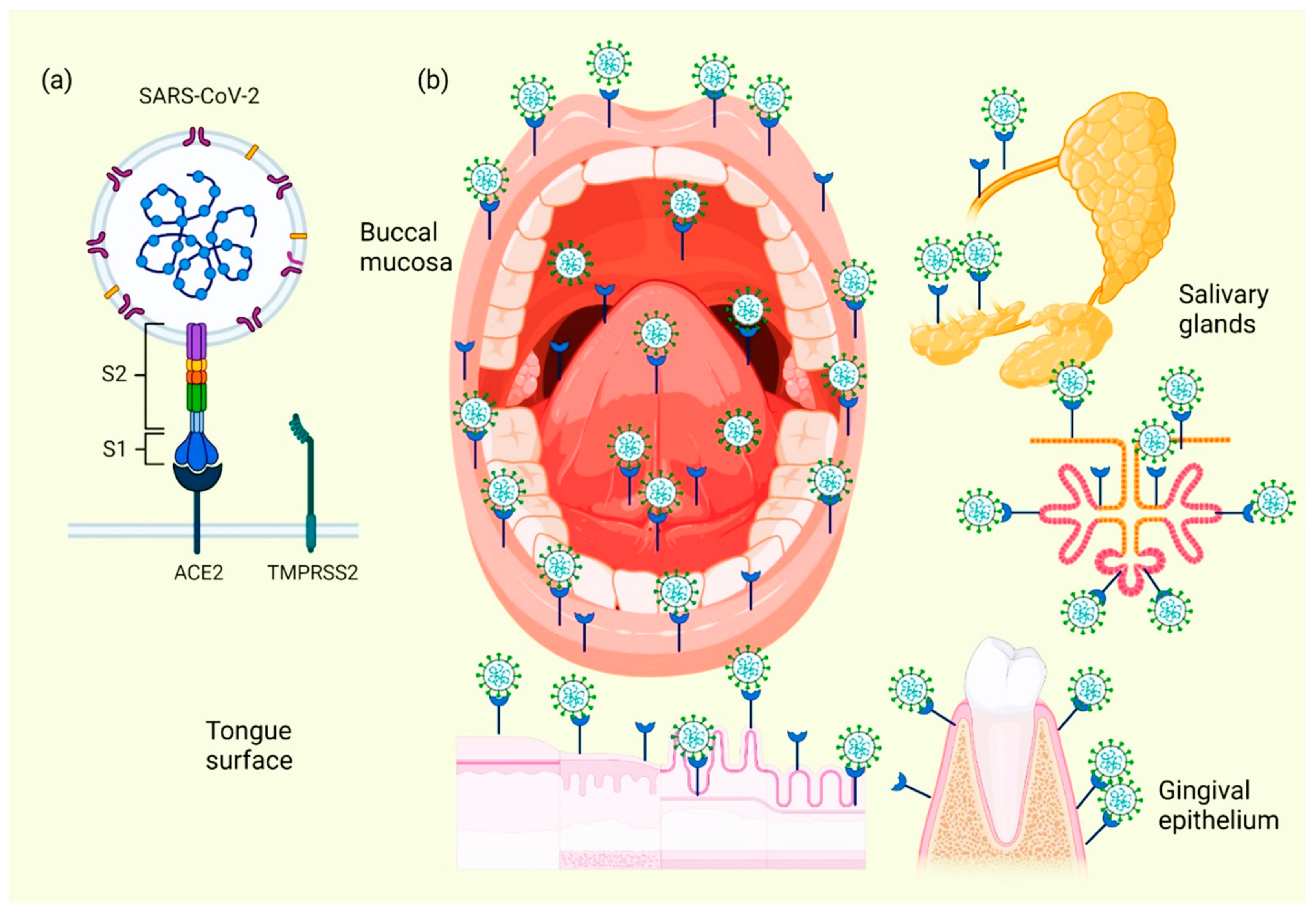
Figure 2. Location of ACE2 on oral cavity and its interaction with the SARS-CoV-2. Binding between the SARS-CoV-2 S protein and the ACE2 protein allows entry of the coronavirus, which subsequently allows its replication and the immediate activation of a possible innate immune response against the virus, including the infiltration of a myriad of immune cells and the subsequent production of many proinflammatory cytokines. This triggers the manifestation of symptoms and signs in the oral cavity of patients with COVID-19 (a). Various spaces and surfaces in the oral cavity where the virus and its receptors are detected, such as the oral mucosa, periodontal tissues, salivary glands, and tongue (b).
Cellular analyses of ACE2 expression as a SARS-CoV-2 entry factor revealed that no oral epithelial subpopulations are at particular risk. The ACE2 receptor was detected in nine oral epithelial cell groups, including basal 1–3, basal cyclic, salivary gland ducts, serous salivary glands, and mucous salivary glands, indicating that multiple oral epithelial cell subpopulations are prone to infection [39]. Coexpression of the most important entry factors ACE2 and TMPRSS2 in mucosal and salivary gland epithelial cells was rare in salivary gland acini and ducts [40]. The clinical development of chemosensitivity disorders usually occurs at the beginning of the infection phase in which the first symptoms are already present, usually within the first three days [41]. Two theories have been described on the pathophysiological factors causing dysgeusia and loss of olfaction in the course of COVID-19 infection: the first theory indicates that SARS-CoV-2 infects neurons using active cellular transport to gain access to the central nervous system [42]. La segunda teoría sugiere que estas disfunciones se deben a la inhibición del receptor ACE2; ya se ha informado que los inhibidores de esta proteína inducen ageusia a través de un mecanismo complejo que involucra el canal de sodio presente en las papilas gustativas y el receptor acoplado a proteína G; tras la interacción entre el SARS-CoV-2 y los receptores de la célula huésped, estos últimos se inactivan, lo que provoca la pérdida de la señalización química del gusto en sus potenciales de acción y, por lo tanto, de la correcta percepción sensorial del gusto [ 41 ] .
This entry is adapted from the peer-reviewed paper 10.3390/ijerph191811383
References
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177.
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854.
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278.
- Galvan Casas, C.; Catala, A.; Carretero Hernandez, G.; Rodriguez-Jimenez, P.; Fernandez-Nieto, D.; Rodriguez-Villa Lario, A.; Navarro Fernandez, I.; Ruiz-Villaverde, R.; Falkenhain-Lopez, D.; Llamas Velasco, M.; et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020, 183, 71–77.
- Al-Khatib, A. Oral manifestations in COVID-19 patients. Oral Dis. 2021, 27 (Suppl. 3), 779–780.
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, CD013705.
- Bemquerer, L.M.; de Arruda, J.A.A.; Soares, M.P.D.; Mesquita, R.A.; Silva, T.A. The oral cavity cannot be forgotten in the COVID-19 era: Is there a connection between dermatologic and oral manifestations? J. Am. Acad. Dermatol. 2021, 84, e143–e145.
- Amorim Dos Santos, J.; Normando, A.G.C.; Carvalho da Silva, R.L.; Acevedo, A.C.; De Luca Canto, G.; Sugaya, N.; Santos-Silva, A.R.; Guerra, E.N.S. Oral Manifestations in Patients with COVID-19: A 6-Month Update. J. Dent. Res. 2021, 100, 1321–1329.
- Sinadinos, A.; Shelswell, J. Oral ulceration and blistering in patients with COVID-19. Evid. Based Dent. 2020, 21, 49.
- Brandao, T.B.; Gueiros, L.A.; Melo, T.S.; Prado-Ribeiro, A.C.; Nesrallah, A.; Prado, G.V.B.; Santos-Silva, A.R.; Migliorati, C.A. Oral lesions in patients with SARS-CoV-2 infection: Could the oral cavity be a target organ? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 131, e45–e51.
- Kamel, A.H.M.; Basuoni, A.; Salem, Z.A.; AbuBakr, N. The impact of oral health status on COVID-19 severity, recovery period and C-reactive protein values. Br. Dent. J. 2021, 24, 1–7.
- Katz, J.; Yue, S.; Xue, W. Dental diseases are associated with increased odds ratio for coronavirus disease 19. Oral Dis. 2022, 28 (Suppl. 1), 991–993.
- Larvin, H.; Wilmott, S.; Wu, J.; Kang, J. The Impact of Periodontal Disease on Hospital Admission and Mortality During COVID-19 Pandemic. Front. Med. 2020, 7, 604980.
- Marouf, N.; Cai, W.; Said, K.N.; Daas, H.; Diab, H.; Chinta, V.R.; Hssain, A.A.; Nicolau, B.; Sanz, M.; Tamimi, F. Association between periodontitis and severity of COVID-19 infection: A case-control study. J. Clin. Periodontol. 2021, 48, 483–491.
- Fabian, T.K.; Hermann, P.; Beck, A.; Fejerdy, P.; Fabian, G. Salivary defense proteins: Their network and role in innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320.
- Diamond, G.; Beckloff, N.; Ryan, L.K. Host defense peptides in the oral cavity and the lung: Similarities and differences. J. Dent. Res. 2008, 87, 915–927.
- Walker, D.M. Oral mucosal immunology: An overview. Ann. Acad. Med. Singap. 2004, 33, 27–30.
- Pivarcsi, A.; Bodai, L.; Rethi, B.; Kenderessy-Szabo, A.; Koreck, A.; Szell, M.; Beer, Z.; Bata-Csorgoo, Z.; Magocsi, M.; Rajnavolgyi, E.; et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int. Immunol. 2003, 15, 721–730.
- Cook, D.N.; Pisetsky, D.S.; Schwartz, D.A. Toll-like receptors in the pathogenesis of human disease. Nat. Immunol. 2004, 5, 975–979.
- Palm, N.W.; Medzhitov, R. Pattern recognition receptors and control of adaptive immunity. Immunol. Rev. 2009, 227, 221–233.
- de Koning, H.D.; Rodijk-Olthuis, D.; van Vlijmen-Willems, I.M.; Joosten, L.A.; Netea, M.G.; Schalkwijk, J.; Zeeuwen, P.L. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: Upregulation of dectin-1 in psoriasis. J. Investig. Dermatol. 2010, 130, 2611–2620.
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974.
- DePaolo, R.W.; Kamdar, K.; Khakpour, S.; Sugiura, Y.; Wang, W.; Jabri, B. A specific role for TLR1 in protective T(H)17 immunity during mucosal infection. J. Exp. Med. 2012, 209, 1437–1444.
- Blander, J.M.; Sander, L.E. Beyond pattern recognition: Five immune checkpoints for scaling the microbial threat. Nat. Rev. Immunol. 2012, 12, 215–225.
- Haynes, B.F.; Soderberg, K.A.; Fauci, A.S. Introduction to the Immune System. In Harrison’s Principles of Internal Medicine, 19th ed.; Kasper, D., Fauci, A., Hauser, S., Longo, D., Jameson, J.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2014.
- Kouketsu, A.; Sato, I.; Abe, S.; Oikawa, M.; Shimizu, Y.; Takahashi, T.; Kumamoto, H. Detection of human papillomavirus infection in oral squamous cell carcinoma: A cohort study of Japanese patients. J. Oral Pathol. Med. 2016, 45, 565–572.
- Nayyar, S.S.; Thiagarajan, S.; Malik, A.; D’Cruz, A.; Chaukar, D.; Patil, P.; Alahari, A.D.; Lashkar, S.G.; Prabhash, K. Head and neck squamous cell carcinoma in HIV, HBV and HCV seropositive patients—Prognosis and its predictors. J. Cancer Res. Ther. 2020, 16, 619–623.
- Ottria, L.; Lauritano, D.; Oberti, L.; Candotto, V.; Cura, F.; Tagliabue, A.; Tettamanti, L. Prevalence of HIV-related oral manifestations and their association with HAART and CD4+ T cell count: A review. J. Biol. Regul. Homeost. Agents 2018, 32, 51–59.
- Ceballos-Salobrena, A.; Gaitan-Cepeda, L.A.; Ceballos-Garcia, L.; Lezama-Del Valle, D. Oral lesions in HIV/AIDS patients undergoing highly active antiretroviral treatment including protease inhibitors: A new face of oral AIDS? AIDS Patient Care STDs 2000, 14, 627–635.
- Falschlehner, C.; Schaefer, U.; Walczak, H. Following TRAIL’s path in the immune system. Immunology 2009, 127, 145–154.
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. New Engl. J. Med. 1999, 340, 448–454.
- Oved, K.; Cohen, A.; Boico, O.; Navon, R.; Friedman, T.; Etshtein, L.; Kriger, O.; Bamberger, E.; Fonar, Y.; Yacobov, R.; et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PLoS ONE 2015, 10, e0120012.
- Scully, C.; Diz Dios, P. Orofacial effects of antiretroviral therapies. Oral Dis. 2001, 7, 205–210.
- Ruiz-Garbajosa, P.; Canton, R. COVID-19: Impact on prescribing and antimicrobial resistance. Rev. Esp. Quimioter. 2021, 34 (Suppl. 1), 63–68.
- Gloster, A.T.; Lamnisos, D.; Lubenko, J.; Presti, G.; Squatrito, V.; Constantinou, M.; Nicolaou, C.; Papacostas, S.; Aydin, G.; Chong, Y.Y.; et al. Impact of COVID-19 pandemic on mental health: An international study. PLoS ONE 2020, 15, e0244809.
- Bajaj, N.; Granwehr, B.P.; Hanna, E.Y.; Chambers, M.S. Salivary detection of SARS-CoV-2 (COVID-19) and implications for oral health-care providers. Head Neck 2020, 42, 1543–1547.
- Sakaguchi, W.; Kubota, N.; Shimizu, T.; Saruta, J.; Fuchida, S.; Kawata, A.; Yamamoto, Y.; Sugimoto, M.; Yakeishi, M.; Tsukinoki, K. Existence of SARS-CoV-2 Entry Molecules in the Oral Cavity. Int. J. Mol. Sci. 2020, 21, 6000.
- Jermy, M.C.; Spence, C.J.T.; Kirton, R.; O’Donnell, J.F.; Kabaliuk, N.; Gaw, S.; Hockey, H.; Jiang, Y.; Zulkhairi Abidin, Z.; Dougherty, R.L.; et al. Assessment of dispersion of airborne particles of oral/nasal fluid by high flow nasal cannula therapy. PLoS ONE 2021, 16, e0246123.
- Huang, N.; Perez, P.; Kato, T.; Mikami, Y.; Okuda, K.; Gilmore, R.C.; Conde, C.D.; Gasmi, B.; Stein, S.; Beach, M.; et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med. 2021, 27, 892–903.
- Vieira Braga, F.A.; Kar, G.; Berg, M.; Carpaij, O.A.; Polanski, K.; Simon, L.M.; Brouwer, S.; Gomes, T.; Hesse, L.; Jiang, J.; et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 2019, 25, 1153–1163.
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020, 42, 1560–1569.
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261.
This entry is offline, you can click here to edit this entry!
