Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
An overview of the physiological role of the principal body fluids in human health is discussed, with an emphasis on key aspects of the structure and functions of macrocirculation and microcirculation. The lymph and the lymphatic system are described in detail, as well as blood flow in the respiratory system, the digestive system, the brain, and the eye. The urinary system, the fluids within the gastrointestinal tract, cerebrospinal fluid, serous body fluids, synovial fluid, and other relevant human fluids are also concisely discussed.
- circulatory system
- lymph
- blood
- biofluids
- body fluids
1. Circulatory System
Simple unicellular organisms absorb the nutrients they need from their immediate environment, and the waste they produce is excreted into the same surroundings. In multicellular organisms, such as human beings, each cell acts as a unicellular entity, and its immediate environment is contained within the body. Organ cells exchange metabolic materials and waste via cell transport or diffusion, and the circulatory system is responsible for “circulating” these essential components throughout the body [1].
The circulatory system (also known as the cardiovascular system or the vascular system) circulates ~5 L of blood at a rate of ~5 L/m under normal resting conditions [2]. The circulatory system comprises the heart, which consists of two pulsatile pumps in series; the arteries, which carry blood and metabolic substrates from the heart; the capillaries, where the exchange of metabolic substrates and waste products with living tissues occurs; the veins, which carry deoxygenated blood; and the lymphatic vessels, which collect the extracellular fluid and return it to circulation.
The vascular network of the circulatory system is organized into complex structures in the form of hierarchal trees with varied branching configurations, designed to fulfil its equally complex tasks. Large arteries (>6 mm) are responsible for carrying oxygenated blood to smaller arteries (1–6 mm), which then source this supply to the arteriolar network (100–1000 μm) to finally reach the capillary beds (10–15 μm). Venules drain the deoxygenated blood from capillaries into larger veins, eventually reaching the heart [3].
The circulatory system is also essential in homeostatic tasks, such as the regulation of body temperature, the adjustment of the supply of oxygen and nutrients in different physiological situations, and humoral communication throughout the body [1].
There are three subsystems within the circulatory system [4]: (i) the systemic circulation, which receives oxygenated blood from the aorta and whose task is to divert it to the systemic capillaries. (ii) the pulmonary circulation, which is irrigated by the pulmonary artery and feeds the pulmonary capillaries; and (iii) the coronary circulation, which supplies the blood that the heart muscle (myocardium) needs to be able to supply blood to the rest of the body.
Although the coronary circulation is the smallest subsystem by blood volume, its proper function is obviously critical. According to [5], about one-third of the inhabitants of Western countries over the age of 35 years die from coronary artery disease; likewise, almost all elderly people experience some alteration of the coronary circulation.
The general path of the circulatory system begins in the left heart, which supplies oxygenated blood to the aorta at a relatively high pressure; the blood is then distributed to smaller arteries and then, finally, to the systemic capillaries, where oxygen is exchanged for waste carbon dioxide with the surrounding tissues. The deoxygenated blood containing waste products continues its path by flowing into the veins and, eventually, reaching the vena cava, which carries it to the right heart. From here, deoxygenated blood flows into the pulmonary artery, which progressively divides into smaller arteries, arterioles, and, eventually, into the pulmonary capillaries that surround the pulmonary alveoli, where the carbon dioxide carried in the haemoglobin of the red blood cells (RBCs) is exchanged for oxygen (which will also be carried in the haemoglobin) as part of the breathing process. Reoxygenated blood flows then from the lungs back to the left heart through the pulmonary veins, thus completing the cycle, as illustrated in Figure 1.
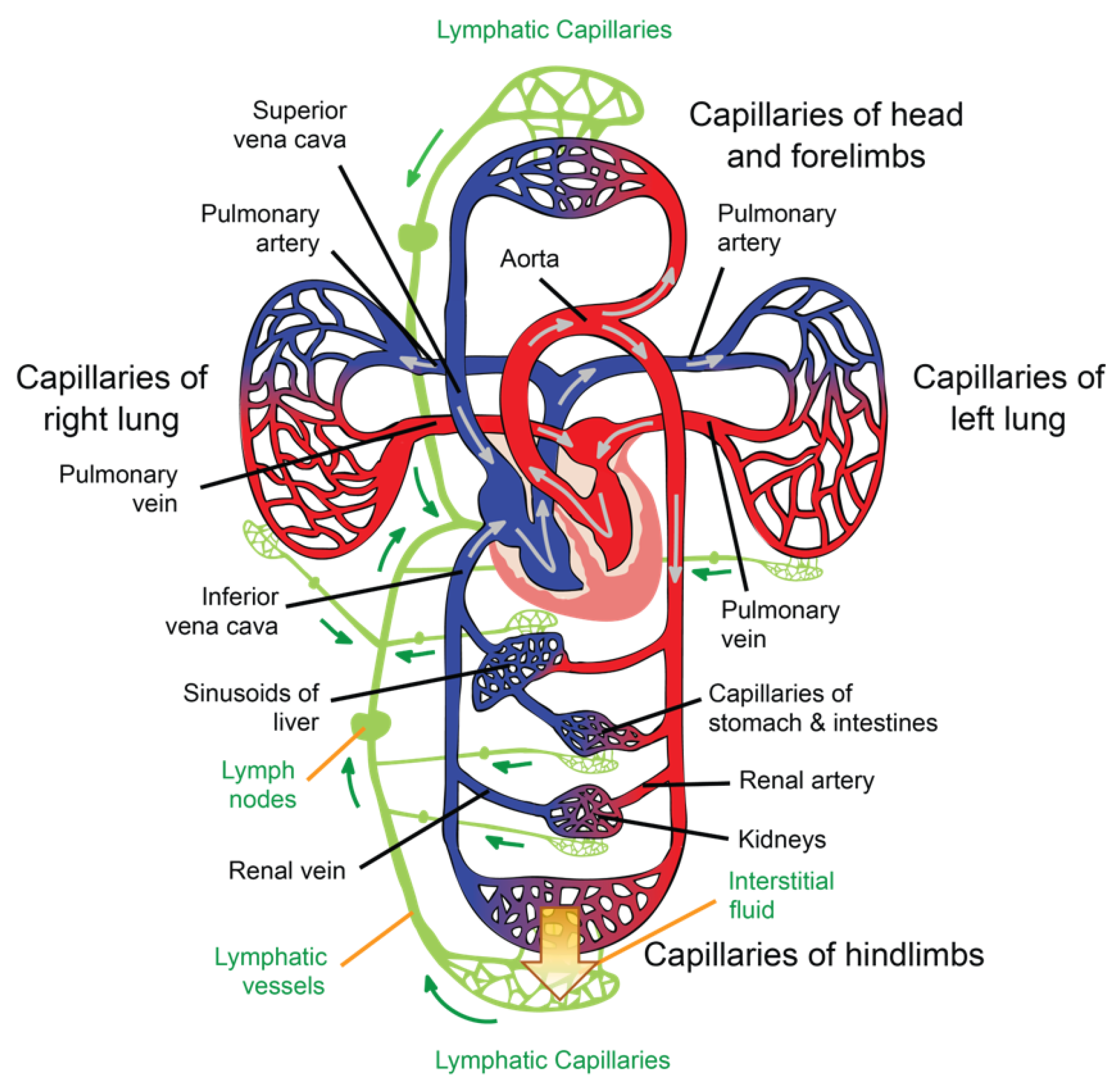
Figure 1. Illustration of the circulatory and lymphatic systems.
1.1. Lymph and the Lymphatic System
The role of the lymphatic system in maintaining health is critical. In the heart, for example, cardiac oedema, inflammation, and fibrosis can be caused by a lymphatic blockage or dysfunction in the cardiac lymphatic vessels [6]. Therefore, in this subsection, we highlight this role by providing a detailed description of lymph and the lymphatic system.
Interstitial fluid (ISF) is produced by extracellular fluid generated in most tissues through a filtration process, which occurs when hydrostatic pressure in the capillary exceeds the opposite intravascular colloid osmotic pressure of plasma proteins [7]. ISF is a clear biofluid that contains solutes, proteins, lipids, waste substances from the metabolism of different tissues, and foreign materials from the interstitial spaces. As is illustrated in Figure 1, the lymphatic system is responsible for draining ISF from the interstitial spaces into the circulatory system. Once ISF enters a lymphatic capillary, it is called lymph [4].
According to [4], a volume of fluid equal to the total volume of plasma in a healthy human is filtered from the blood into the tissues each day; therefore, the lymphatic vessels collect, in normal conditions, ~3 L of ISF each day and return it to the venous system via lymph flow.
Like other extracellular fluids, lymph contains electrolytes and, in addition, a considerable amount of protein (40–60 g L−1), albumin being the main portion (23–34 g L−1). In peripheral lymph, the white blood cell count is ~5 × 108 cells per litre, of which half are lymphocytes. On the other hand, lymph entering the thoracic duct contains ~1 × 1010 lymphocytes per litre, emphasizing the role of the lymphatic system in the immune response. Lymph drainage maintains the ISF protein level at ~15 g L−1, which is, normally, low enough to prevent oedema. ISF averages a colloid osmotic pressure of ~5 mmHg, ensuring a significant gradient between the interstitial tissues and capillary lumen, favouring tissue water reabsorption [8].
The lymphatic network is responsible for three main functions: (i) it maintains circulatory homeostasis by receiving ultrafiltrate from blood capillaries and an excess of protein from the fluid within the interstitial space, and then returns them to the venous system; (ii) lymphoid tissue in the intestinal tract absorbs digested fat, thus aiding in nutrition; (iii) the lymphatic system is a key player in defence, as bacteria, foreign antigens, and lymphocytes found in the interstitium drain with lymph to regional lymph nodes, activating the immune system [6].
Subcutaneous lymph-collecting vessels are commonly known as lymphatics and are found in almost all parts of the body that contain blood vessels. They begin in the superficial dermis but are not present in the epidermis [9], and they lie beneath the mucosa and serous lining of body cavities and major organs [6]. The junctions between the lymphatic cells are not tight junctions; overlapping cells form a freely permeable system that allows for the entry of ISF into the lumen of the lymphatic vessels. In the interstitial space, the lymph passes from the initial lymphatics to the lymphatic capillaries. Lymphatics are described as blind bulbs 5–50 mm wide [8], whose intimae are formed by elongated, single-layer endothelial cells supported by an elastic membrane. Their media are made up of transverse smooth muscle and fine elastic fibres, and the adventitia consists of connective tissue mixed with smooth muscle cells, fine blood vessels, and a fine nerve plexus [9].
Lymphatic capillaries are present in most body tissues and range from 20 to 70 μm in diameter. The palms of the hands, fingers, the soles of the feet, and toes are richly provided with such structures. These capillaries drain lymph to pre-collectors of up to 300 μm in diameter that contain valves and join together to form lymphatic vessels (see Figure 1), which are also valved [9]. The segments of vessels between the two valves are known as lymphangia. Smooth muscles in the walls of the lymphatic vessels cause the lymphangia to contract sequentially, allowing lymph to flow toward the thoracic region [8].
In the limbs, the superficial lymphatic vessels are located just below the skin, accompanying the superficial veins. Their perforators pass to the deep lymphatic vessels through the deep fascia. The deep lymphatic vessels are less numerous; however, they are larger than the superficial vessels and accompany the deep blood vessels [9].
Lymphatic vessels from the lower limbs enter the posterior abdominal wall after exiting the inguinal lymph nodes and, eventually, reach the cisterna chyli. From here, lymph enters the thoracic duct, which also receives lymph flow from the left side of the thorax, left arm, and the left side of the head and neck. The thoracic duct enters the venous system at the junction of the left subclavian vein and the left internal jugular vein. The right lymphatic duct drains lymph from the right side of the thorax, the right arm, and the right side of the head and neck [8].
Unlike the circulatory system, there are no active pumps to assist the lymphatic system; the movement of ISF from the surroundings of tissues through the lymphatic vessels is passive; a cycle of compression/relaxation causes lymphatic vessels to draw lymph. The pressure in the interstitial fluid around a capillary opens the overlapping cells of the lymphatic bulbs [4], and the endothelial array of the lymphatic capillary containing myoendothelial fibres contracts rhythmically, thus allowing the vessels to pump lymph toward larger lymphatic vessels, which, in turn, contain small bicuspid valves that ensure that the lymph is pumped in the right direction. Furthermore, the junctions between the endothelial cells act as a valve mechanism, which prevents lymph from returning to the interstitial space. After contraction, the connective tissue fibres that anchor the endothelial cells pull the lymphatic capillary back open, prepared to be refilled with more interstitial fluid [8].
Lymphatic pressures are only a few mmHg in the bulbs and smallest lymphatic vessels, but they can be as high as 25 mmHg in larger lymphatic vessels [8]. Lymphatic valves are responsible for this progression from low to high pressures [4].
At intervals along the lymphatic vessels, there exist lymph nodes. Lymph flows through the lymph nodes to reach the more proximal lymphatic vessels. Essentially, they consist of lymphoid follicles between 0.2 mm and 20 mm in length through which lymph is filtered [8]. There are about 500–600 lymph nodes clustered in the axilla, inguinal region, neck, pelvis, mediastinum, and para-aortic space [9].
A lymph node has a capsule leading to a trabecula, which divides the node into follicles containing sinusoids. Lymph nodes have a cortex into which the afferent lymphatics drain and a medulla containing lymphoid tissue that extends to the hilum, where the efferent lymphatics exit. Blood vessels enter and exit the hilum, but the arterioles, capillaries, and venules remain separate from the lymph that passes through the sinusoids within the lymph node [9].
As we mentioned before, lymph nodes facilitate the interaction between lymph and the immune system, being a key factor in the protection of the organism against infections and tumours [8].
1.2. Macrocirculation and Microcirculation
When blood flows through the aorta, which has an average diameter of 25 mm, the RBCs are small enough (~8 μm) for blood to be considered a homogeneous fluid. As the blood approaches the capillaries, which are similar in size to an RBC, a change between macrocirculation and microcirculation occurs. Capillary blood vessels are so narrow that their walls scrape the RBC membranes, so they flow in a single row. Although the frontier between these two subsystems is sometimes debated, the distinction between micro- and macrocirculation tends to be based on the Reynolds number and the Womersley number; if their value is much smaller than one, then the inertial force can be ignored, and the flow is considered microcirculation. Conversely, if both numbers are much greater than one, the fluid viscosity can be ignored and the flow is said to be macrocirculation. In the middle of this limit, the distinction becomes difficult to resolve and becomes inconsequential [10]. Under a different criterion, [4] microcirculation is defined as blood flow in vessels with a diameter of 100 μm or less. These microvessels include arterioles, metarterioles, capillaries, and venules. The authors of [4] also refer to the fact that almost all cells within the human body are close to a microvessel.
Microcirculation deals with ~10% of the circulating blood volume (in healthy conditions), and is the part of the circulatory system that is in direct contact with parenchymal cells [11]. Blood flow in microcirculation is usually referred to as perfusion, which is defined as the blood flow rate per unit of tissue volume. Its role in the exchange of water, hormones, nutrients, gases, and metabolic waste products between blood and cells is critical; in addition, microcirculation is responsible for regulating vascular resistance, the transport of heat, and the distribution of pharmaceuticals [4][12].
Capillaries are tubular structures with a diameter of 4–9 μm and one-cell-layer thick walls (~0.5 μm) made of highly permeable endothelial cells [4]. As we mentioned before, filtration occurs when a fluid permeates the capillary walls and moves into the interstitial fluid of a tissue due to capillary hydrostatic pressure; the opposite process, that is, the movement of fluid from the interstitial fluid back into the capillaries, is called reabsorption, and it occurs due to the osmotic pressure (also referred to as oncotic pressure) caused by the difference in the solute-to-water concentrations in the blood and tissue fluid [12]. After leaving the capillaries, most blood returns to venules and later to the veins; however, ~10% of the fluid leaving the capillaries enters the lymphatic capillaries and returns to the blood through the lymphatic system [4], as is illustrated in Figure 1.
The study and knowledge of tissue hemodynamics are of great interest in many clinical areas that use it as a diagnostic tool for various pathologies related to the circulatory system. Perfusion is a key parameter in, for example, determining the viability of an organ or tissue before transplantation; the prognosis of patients suffering from ischemic heart disease; the determination of brain damage in stroke victims; and determining the condition of diseased organs such as kidneys, liver, pancreas, and lungs [13][14][15]. Perfusion is also considered in some drug studies, in cancer treatment, and in the laser photocoagulation of tumours. However, despite its mentioned clinical importance, there is no ideal way to measure perfusion to date [16][17].
1.3. Histology of the Skin and Cutaneous Blood Flow
The easiest organ to assess through any device aimed to evaluate a biological flow profile is, obviously, the skin and its capillary network, since it is the outermost organ of the human body. In addition, the skin is the first barrier that a sensor must deal with before reaching any target of interest within the body. Since the angiogenesis process—the growth of new capillaries from pre-existing vessels—is a notable factor in cutaneous melanoma [18], one of the key uses of flow sensors is in the diagnosis and prognosis of skin cancer [19].
The integumentary system comprises the skin and its appendages (hair–hair follicles, nails, sudoriferous glands, and sebaceous glands), and is the external layer that covers the human body from head to toe, thus being the first protective barrier against harmful elements, such as fire, ultraviolet (UV) rays, dust, microbes, acids, etc. According to [20], for a 70 kg man, the extent of skin is ~1.7 m2 and its weight is ~3.86 kg, accounting for ~5.5% of the total body mass.
The skin is a stratified, heterogeneous, and anisotropic medium composed of two main layers joined together: the epidermis and dermis. Contrary to popular belief, the layer beneath the dermis, called the hypodermis or subcutaneous layer, is not considered part of the skin [21]. The thickness of each layer is different and, additionally, they are made of different solid materials saturated with fluids [22]. Their functions are different from each other, as each layer is seen as a system in itself. The main characteristics of these layers are described below.
Epidermis
The epidermis is the superficial layer of the skin, ranging in thickness from 0.07 to 0.12 mm along the surface of the body [23]. It is a keratinized, stratified squamous epithelium that is not innervated and is avascular; the nutrients that the epidermis requires arrive from the dermis by diffusion, and the removal of metabolic waste products also depends on the blood vessels of the dermis [21].
The epidermis is composed of up to five layers, which, viewed from the outside inwards, are: the stratum corneum, stratum lucidum (found only on palms and soles), stratum granulosum, stratum spinosum, and stratum basale. Collectively, the stratum spinosum and the stratum basale are usually referred to as stratum malpighi.
The epidermis contains four main types of cells: keratinocytes (90%); melanocytes (8%), which produce the pigment of the skin (melanin); Langerhans cells; and Merkel cells [21].
Dermis
The dermis is below the epidermis and above the hypodermis. Its thickness is between 1 and 4 mm and is composed of two layers: the papillary dermis and reticular dermis [23]. The main function of the dermis is to provide nutrients and physical support to the epidermis [21].
The papillary dermis is the upper layer, and it comprises about 10% of the entire dermal thickness. It contains thin collagen fibrils of 20–40 nm in diameter packed into thicker collagen fibres ranging from 0.3 to 3.0 mm in diameter [23]. In addition, the papillary dermis contains the nerves and capillaries that nurture the epidermis [21].
The reticular dermis lies below the papillary layer and is made up of strong connective tissue containing collagen and elastic fibres [21]; its collagen fibrils, of 60–100 nm in diameter, are composed, primarily, of type I collagen and are organized into fibres ranging from 10 to 40 mm in diameter [23].
In the average young adult, the collagen of the papillary dermis is similar to a randomly oriented fine fibre feltwork, whereas that of the reticular layer consists of large, wavy, and randomly oriented bundles of collagen that are loosely interwoven [23].
Dermal–Epidermal Junction
As explained in [24], the structural integrity of the skin depends on the dermal–epidermal junction, which is a complex network of extracellular matrix macromolecules that interconnect both layers.
The thickness of the dermal–epidermal junction is ~100 nm and is characterized by a wave-shaped pattern arising from the epidermal rete ridges to the papillary dermis; such elevations indent the epidermis, thus increasing the surface that is in contact with both layers, strengthening the dermal–epidermal connectivity and keeping, consequently, the dermis and epidermis layers firmly connected. Rete ridges surround the dermal papillae, which are small protruding extensions of the papillary dermis embedded within the epidermis. On a macroscopic level, fingerprints are a manifestation of this undulating pattern of ridges and furrows [24].
When the epidermis and dermis separate as a result of shear or friction forces, body fluids, such as lymph, serum, plasma, blood, or pus, can accumulate between the two layers, forming what is commonly known as a blister [21].
Vascularization of Human Skin
The skin receives vascular supply through two main networks of cutaneous arteries:
- (i)
-
The superficial vascular plexus, which is a network of blood vessels located at the uppermost level of the dermis. From a histological point of view, this plexus marks the junction between the papillary and reticular dermis. The superficial plexus is composed of anastomosing small-calibre arterioles that branch off into capillaries, which extend into dermal papillae to supply the boundaries between the epidermis and dermis and envelop adnexal structures [25]. Each dermal papilla is provided with at least one capillary loop [24]. In Figure 2, an image of a pig skin sample—obtained through a 50× microscope objective and a lateral scan at different depths—shows an example of a capillary loop.
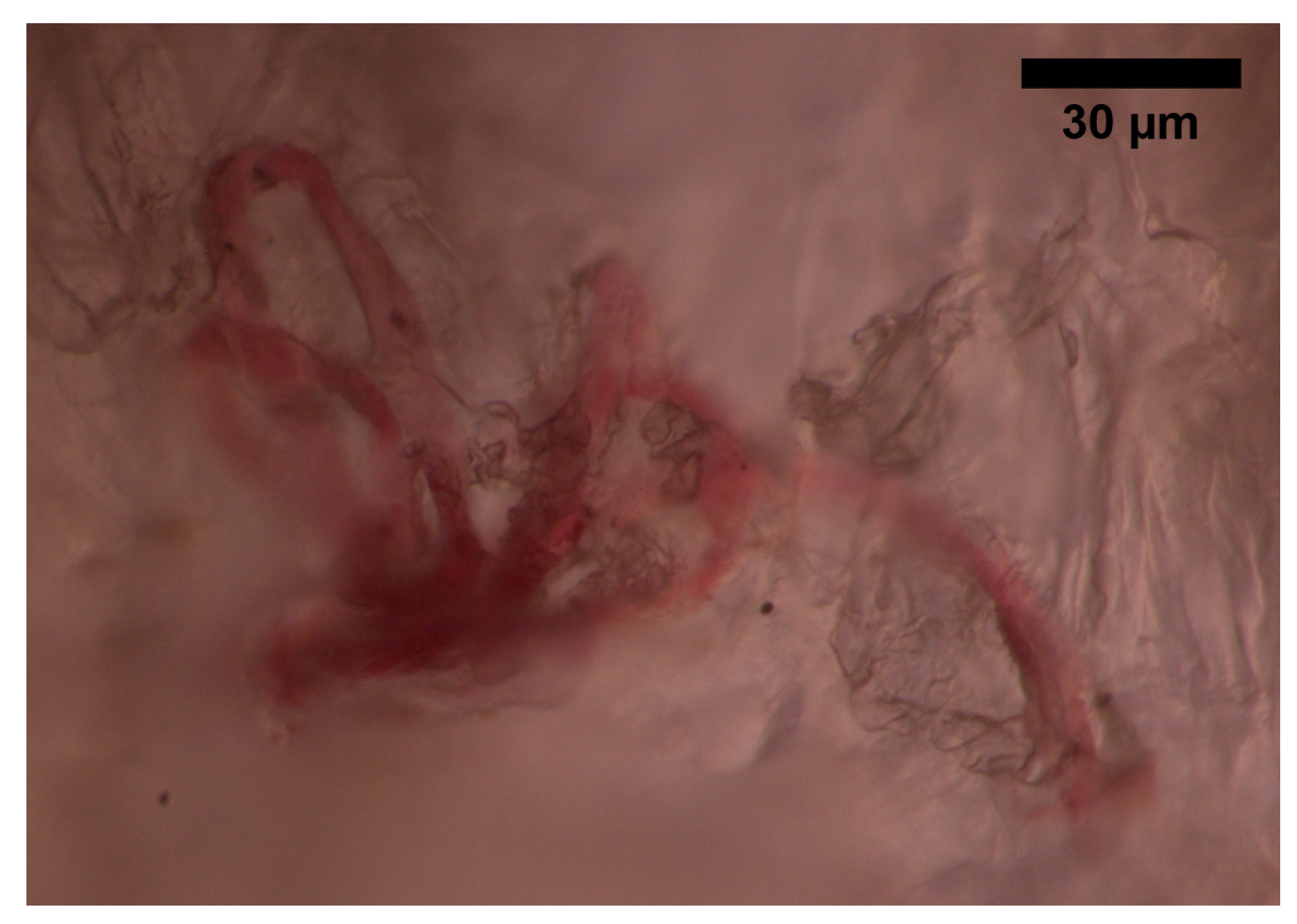 Figure 2. Image of a capillary loop from a sample of pig skin obtained with an optical microscope and an M-Plan APO 50 × 0.55 NA microscope objective.
Figure 2. Image of a capillary loop from a sample of pig skin obtained with an optical microscope and an M-Plan APO 50 × 0.55 NA microscope objective.
- (ii)
-
The deep vascular plexus, which is found at the joint between the dermis and hypodermis. It is a histological landmark that delimits the reticular part of the dermis from the hypodermis. This plexus is composed of medium-calibre vessels that emerge from larger vessels crossing the adipose septae of the hypodermis and is connected to the superficial vascular plexus by vertically oriented vessels [25]. Small tributaries sprout from this plexus to supply sweat glands, hair follicles, and other structures within the dermis [21].
Although the hypodermis is not considered part of the skin, the vascularization of this layer plays an important role in the blood supply of the skin because the subcutaneous arterial network has a larger extension and runs parallel to the cutaneous network, thus influencing the skin perfusion flow profile. Moreover, all the small arterial branches of the skin come from the hypodermis [26].
1.4. Blood Flow in the Respiratory System
The circulatory system and the respiratory system are strongly connected (see Figure 1), as the pulmonary circulation supplies blood to the alveoli for gas exchange. The bronchial system and the trachea, on the other hand, are fed by the bronchial circulation, which has high pressure and high oxygen content [27].
1.5. Blood Flow in the Digestive System
The digestive tract provides the body with a constant supply of water, nutrients, and electrolytes. To do this, the gastrointestinal blood circulation distributes the absorbed substances through the gastrointestinal organs (e.g., oesophagus, diaphragm, stomach, spleen, liver, pancreas) and supplies essential oxygen for the absorptive and secretory functions of the gastrointestinal tissues, especially for those in the layer of the gut, such as the mucosa [27].
The blood vessels of the digestive system are known as the splanchnic circulation. After passing through the stomach, intestines, spleen, and pancreas, blood reaches the liver via the portal vein. Inside the liver, blood circulates through millions of sinusoidal capillaries, leaving the liver via the hepatic veins into the vena cava, which carries it back to the heart [27].
1.6. Main Aspects of Blood Flow in the Brain and Eye
According to [27], the brain receives blood mainly from the left and right carotid arteries and, secondly, from the left and right vertebral arteries; the blood flow in the brain represents ~14–15% of total blood circulation. A branch of the carotid artery, the ophthalmic artery, enters the orbit of the eye through the optic canal and is divided into other branches that supply blood to the structures of the orbit, such as the lacrimal artery, which supplies the lacrimal gland, muscles, the anterior ciliary arteries, and the eyelid laterals; the long and short posterior ciliary arteries, which carry blood to the internal structures of the eyeball; the central retinal artery, which enters the optic nerve, dividing into many branches over the internal surface of the retina; the muscular arteries, which supply blood to the intrinsic muscles of the eyeball; the medial palpebral arteries, which feed the medial area of the lower and upper eyelids; the anterior ethmoidal artery, which supplies blood to the lateral wall and the nasal septum; the posterior ethmoidal artery, which delivers blood to the ethmoidal cells and nasal cavity; the dorsal nasal artery, which supplies blood to the upper surface of the nose; the supraorbital artery, which carries blood to the scalp and forehead; and the supratrochlear artery, which feeds the forehead.
The branches of the ophthalmic artery mentioned above, and the veins that drain the posterior part of the eyeball, contribute blood to the superior ophthalmic vein; on the other hand, the inferior ophthalmic vein receives tributaries from various muscles, as well as the eyeball posterior part [27].
2. The Urinary System
The circulatory system and blood flow are closely related to the urinary system; adequate blood volume and pressure are necessary for urine formation and, conversely, urine removes metabolic waste products, toxins, and drugs from the circulatory system [28].
The urinary system is made up of the kidneys, which are paired organs located in the lower back responsible for producing urine from the filtration of blood; the ureters, which transport the urine to the bladder; the bladder, where urine is stored; and the urethra, which connects the bladder with the external urethral orifice for urine excretion. For an average adult under normal conditions, daily urine production can range from 600 to 2000 mL/24 h. [28].
The important errands of the urinary system are the regulation of body fluids, maintaining the acid–base balance and the electrolyte balance, the maintenance of blood pressure and erythropoiesis and, of course, the excretion of waste products [28].
Urine contains several metabolites, such as glucose, cholesterol, urate, lactate, oxalate, and ascorbate, which can be analysed to diagnose various disease conditions, e.g., glucose found in urine is associated with diabetes [29][30], while the detection of lactate is used in the metabolic screening of inherited diseases [31][32][33].
3. Fluids within the Gastrointestinal Tract
There are several substances involved in digestion. Parietal cells secrete the glycoprotein gastric intrinsic factor, which is used in the absorption of vitamin B12. They also produce hydrochloric acid, which hydrolyses peptides and disaccharides and transforms the proenzyme pepsinogen into pepsin. In turn, pepsinogen is secreted by peptic cells and catalyses the degradation of proteins to proteases and peptones [28].
Other digestive enzymes secreted by the stomach are lipase, lactase, and peptidase. In addition, goblet cells and mucous glands secrete mucus that prevents the stomach walls from being damaged by acids and enzymatic activity [28].
4. Cerebrospinal Fluid
The capillary blood vessels within the ventricular choroid plexus produce ~70% of the cerebrospinal fluid by means of a combination of active secretion and plasma ultrafiltration. The remaining ~30% is formed by the cerebral/subarachnoid space and the ependymal lining cells of the ventricles. For a healthy adult, the volume of cerebrospinal fluid ranges from 90 to 150 m/L, with a production of 500 mL/24 h. The cerebrospinal fluid exits the ventricles through the foramina and circulates the hemispheres of the brain, descending over the spinal cord toward the nerve roots. The cerebrospinal fluid circulates slowly, allowing for prolonged contact with cells in the central nervous system. Later, in the dural sinuses, the cerebrospinal fluid is reabsorbed by the arachnoid villi [28].
The cerebrospinal fluid serves as a protective element, cushioning and lubricating the brain and vertebral column, avoiding injuries as a result of gravitational or inertial forces. It also exchanges nutrients and metabolic waste products with the brain and spinal cord [28].
5. Serous Body Fluids
This type of fluid, made up of ultrafiltrate plasma, is normally clear and slightly yellowish and is called serous because it resembles serum. The serous fluid is secreted by the serous membrane, which is a smooth tissue membrane lining the organs and walls of the different body cavities. The serous fluid fills the space between the visceral portion (the part that covers the organs) and the parietal portion (the part that lines the body walls) of the serous membrane, acting as a lubricant [28].
The serous cavities include the pericardium, which, in normal conditions, contains less than 50 mL of pericardial serous fluid surrounding the heart; the pleura, which normally contains less than 30 mL of pleural serous fluid around the lungs; and the peritoneum, which accumulates the peritoneal serous fluid (also known as ascites) that surrounds the organs contained within the abdominal cavity [28].
6. Synovial Fluid
Synovial fluid (also called synovia) is the viscous, mucinous substance that lubricates most joints; hence, its analysis is relevant for the diagnosis of the common diseases that affect human joints. Synovial fluid is produced in the synovium, a tissue that lines diarthrotic joints and allows bones to articulate freely [28].
Synovia is a dialysate of plasma that contains levels of glucose and uric acid equivalents to that of plasma and one-third of its protein levels. This dialysate is combined with a mucopolysaccharide synthesized by the synovium. Under normal conditions, synovia is a transparent and very viscous fluid, whose volume within the joints is small, e.g., the knee joint contains ~4 mL of synovia [28].
7. Other Relevant Human Fluids
Semen is made up of various fluids produced in different male organs. The seminal vesicles produce a slightly alkaline fluid that contains citric acid, fructose, potassium, and flavins. This fluid provides spermatozoa (which are formed in the sertoli cells of the seminiferous tubules of the testis) with these essential nutrients and comprises more than half the volume of semen. The prostate gland contributes 20% of the composition of semen, producing a fluid that contains citric acid, acid phosphatase, and proteolytic enzymes. The remaining portion of semen is produced in the urethral glands, bulbourethral glands, and the epididymis [28].
Vaginal secretions are produced in glands located on the cervix and their volume can vary throughout the menstrual cycle. Normally, vaginal secretions are clear mucus that can turn slightly white or pale yellow upon contact with air; abnormal changes in colour, amount, or consistency can be an indicator of different conditions or infections [28].
Amniotic fluid comes from the placenta and surrounds the developing foetus inside a membranous sac known as the amnion. In addition to being a protective element, it plays a key role in the exchange of water, nutrients, and biochemical products between the foetus and the maternal circulation. The composition of amniotic fluid is similar to that of the maternal plasma, with biochemical substances produced by the foetus and a low number of cells from the urinary tract, digestive tract, and skin of the newborn. When the foetal production of urine begins, urine also contributes to the amniotic fluid [28].
This entry is adapted from the peer-reviewed paper 10.3390/s22186836
References
- Mazumdar, J. Biofluid Mechanics; World Scientific: Singapore; River Edge, NJ, USA, 1992; ISBN 978-981-02-0927-8.
- Rubenstein, D.A.; Yin, W.; Frame, M.D. Biofluid Mechanics: An Introduction to Fluid Mechanics, Macrocirculation, and Microcirculation, 2nd ed.; Academic Press Series in Biomedical Engineering; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2015; ISBN 978-0-12-800944-4.
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv. Funct. Mater. 2020, 30, 1910811.
- Waite, L.; Fine, J.M. Applied Biofluid Mechanics; McGraw-Hill: New York, NY, USA, 2007; ISBN 978-0-07-147217-3.
- Pheng, T.S.; Qing, M.S.Z.; Kee, H.W.; Iqbal, M.Z.; Parveen, S.; Aziz, S.; Mohiuddin, G.; Tahir, M. A Review on Prevalence, Causes, Preventions, and Treatments of Coronary Artery Disease. Asian Pac. J. Health Sci. 2017, 4, 104–107.
- Karunamuni, G. (Ed.) The Cardiac Lymphatic System; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-6773-1.
- Ramirez, C. (Ed.) The Lymphatic System: Components, Functions and Diseases; Human Anatomy and Physiology; Nova Biomedical: New York, NY, USA, 2016; ISBN 978-1-63484-689-9.
- Labropoulos, N.; Stansby, G. (Eds.) Venous and Lymphatic Diseases; Taylor & Francis: New York, NY, USA, 2006; ISBN 978-0-8247-2923-3.
- Myers, K.A.; Hannah, P. Manual of Venous and Lymphatic Diseases; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-138-03676-5.
- Fung, Y.C. Biomechanics; Springer: New York, NY, USA, 1990; ISBN 978-1-4757-5913-6.
- Khanna, A.K.; Karamchandani, K. Macrocirculation and Microcirculation: The “Batman and Superman” Story of Critical Care Resuscitation. Anesth. Analg. 2021, 132, 280–283.
- Capillary Exchange|Anatomy and Physiology. Available online: https://courses.lumenlearning.com/nemcc-ap/chapter/capillary-exchange/ (accessed on 21 May 2021).
- Panisello-Roselló, A.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Rolo, A.; Palmeira, C.; Adam, R.; Net, M.; Roselló-Catafau, J. Role of Aldehyde Dehydrogenase 2 in Ischemia Reperfusion Injury: An Update. World J. Gastroenterol. 2018, 24, 2984–2994.
- De Carlis, R.; Di Sandro, S.; Lauterio, A.; Ferla, F.; Dell’Acqua, A.; Zanierato, M.; De Carlis, L. Successful Donation after Cardiac Death Liver Transplants with Prolonged Warm Ischemia Time Using Normothermic Regional Perfusion. Liver Transpl. 2017, 23, 166–173.
- Shapey, I.M.; Muiesan, P. Regional Perfusion by Extracorporeal Membrane Oxygenation of Abdominal Organs from Donors after Circulatory Death: A Systematic Review: Extracorporeal Membrane Oxygenation. Liver Transpl. 2013, 19, 1292–1303.
- Leondes, C.T. (Ed.) Biofluid Methods in Vascular and Pulmonary Systems; Biomechanical Systems; CRC Press: Boca Raton, FL, USA, 2001; Volume 4, ISBN 978-0-8493-9049-4.
- Michael Peters, A. The Precise Physiological Definition of Tissue Perfusion and Clearance Measured from Imaging. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1139–1141.
- Streit, M.; Detmar, M. Angiogenesis, Lymphangiogenesis, and Melanoma Metastasis. Oncogene 2003, 22, 3172–3179.
- Rey-Barroso, L.; Peña-Gutiérrez, S.; Yáñez, C.; Burgos-Fernández, F.J.; Vilaseca, M.; Royo, S. Optical Technologies for the Improvement of Skin Cancer Diagnosis: A Review. Sensors 2021, 21, 252.
- Goldsmith, L.A. My Organ Is Bigger Than Your Organ. Arch. Dermatol. 1990, 126, 301.
- McLafferty, E.; Hendry, C.; Farley, A. The Integumentary System: Anatomy, Physiology and Function of Skin. Nurs. Stand. 2012, 27, 35–42.
- Abellan, M.-A.; Zahouani, H.; Bergheau, J.-M. Contribution to the Determination of In Vivo Mechanical Characteristics of Human Skin by Indentation Test. Comput. Math. Methods Med. 2013, 2013, 814025.
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic Properties of Human Skin and Processed Dermis: Viscoelastic Properties of Human Skin. Ski. Res. Technol. 2001, 7, 18–23.
- Roig-Rosello, E.; Rousselle, P. The Human Epidermal Basement Membrane: A Shaped and Cell Instructive Platform That Aging Slowly Alters. Biomolecules 2020, 10, 1607.
- Marghoob, A.A. (Ed.) Atlas of Dermoscopy, 2nd ed.; Informa Health Care: London, UK, 2012; ISBN 978-0-415-45895-5.
- Zbrodowski, A.; Gajisin, S.; Bednarkiewicz, M.; Gumener, R.; Montandon, D. Blood Supply of Subcutaneous Tissue in the Leg and Its Clinical Application. Clin. Anat. 1995, 8, 202–207.
- Ostadfar, A. Biofluid Mechanics: Principles and Applications; Elsevier/Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2016; ISBN 978-0-12-802408-9.
- Mundt, L.A.; Shanahan, K.; Graff, L. Graff’s Textbook of Urinalysis and Body Fluids, 3rd ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2016; ISBN 978-1-4963-2016-2.
- Morris, L.R. Correlation Between Plasma and Urine Glucose in Diabetes. Ann. Intern. Med. 1981, 94, 469.
- Malone, J.I. The Role of Urine Sugar in Diabetic Management. Arch. Pediatr. Adolesc. Med. 1976, 130, 1324.
- Ketting, D.; Wadman, S.K.; Bruinvis, L.; Sweetman, L. The Occurrence of Lactyl Lactate and Succinyl Lactate in the Urine of Patients Screened for Inherited Metabolic Disease. Clin. Chim. Acta 1985, 146, 29–35.
- Hagen, T.; Korson, M.S.; Wolfsdorf, J.I. Urinary Lactate Excretion to Monitor the Efficacy of Treatment of Type I Glycogen Storage Disease. Mol. Genet. Metab. 2000, 70, 189–195.
- Thirumurugan, A.; Thewles, A.; Gilbert, R.D.; Hulton, S.-A.; Milford, D.V.; Lote, C.J.; Taylor, C.M. Urinary L-Lactate Excretion Is Increased in Renal Fanconi Syndrome. Nephrol. Dial. Transplant. 2004, 19, 1767–1773.
This entry is offline, you can click here to edit this entry!
