Mycobacterium avium subspecies paratuberculosis (MAP) is an environmentally hardy pathogen of ruminants that is transmitted via the fecal-oral route. Transition from subclinical to clinical infection is a dynamic process led by MAP, which survives and replicates in host macrophages. Hallmark clinical symptoms include granulomatous enteritis, watery diarrhea, and significant loss of body condition. Clinical stage disease is accompanied by dysfunctional immune responses and a reduction in circulating vitamin D3. The immunomodulatory role of vitamin D3 in infectious disease has been well established in humans, particularly in Mycobacterium tuberculosis infection. However, significant species differences exist between the immune system of humans and bovines, including effects induced by vitamin D3.
- vitamin D
- Mycobacterium avium subsp. paratuberculosis
- cattle
- macrophage
- PBMC
- endosomal trafficking
1. Johne’s Disease Overview
2. Transmission
3. Immune Responses to MAP
4. Vitamin D
4.1. History
4.2. Metabolism and Signaling
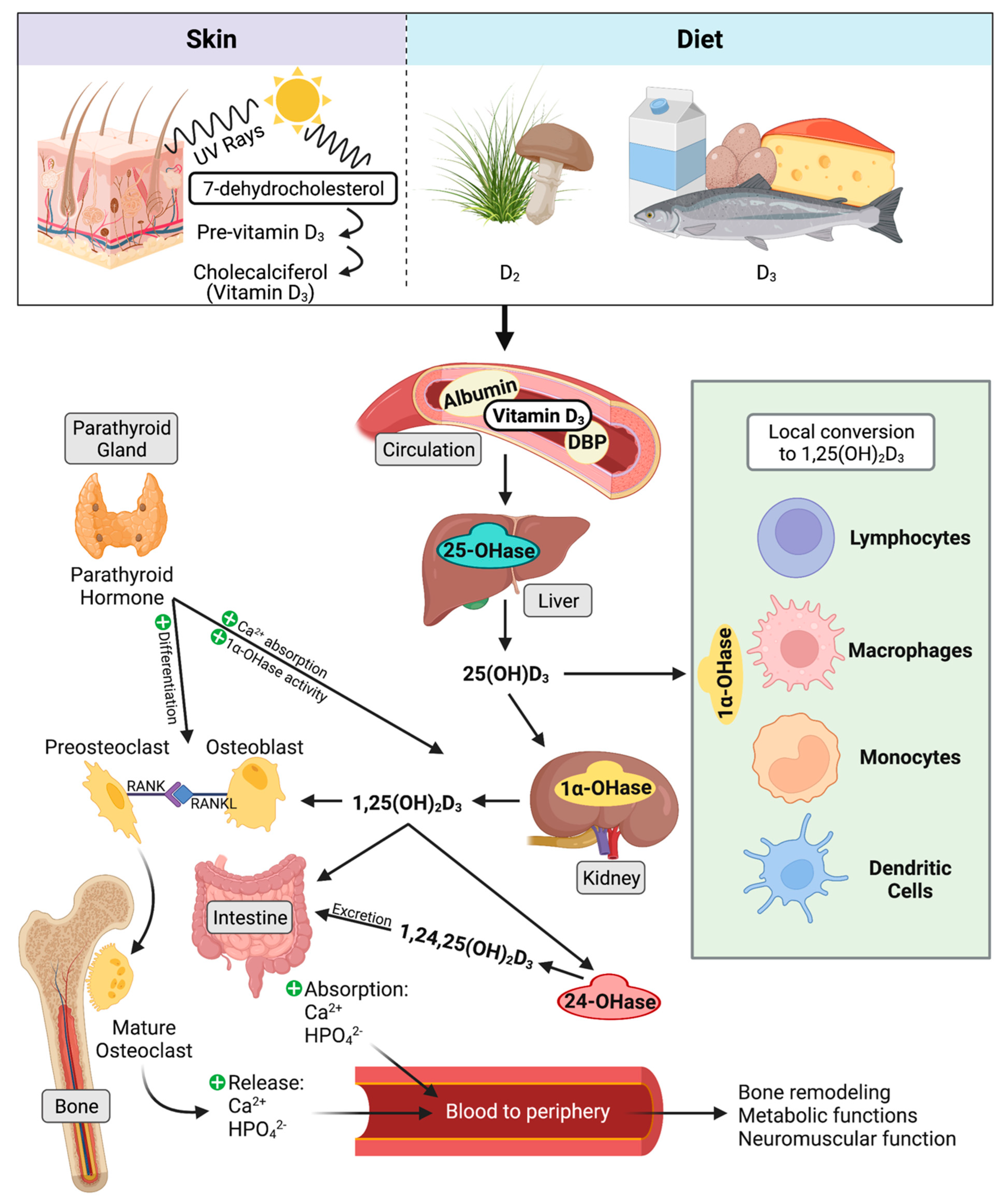
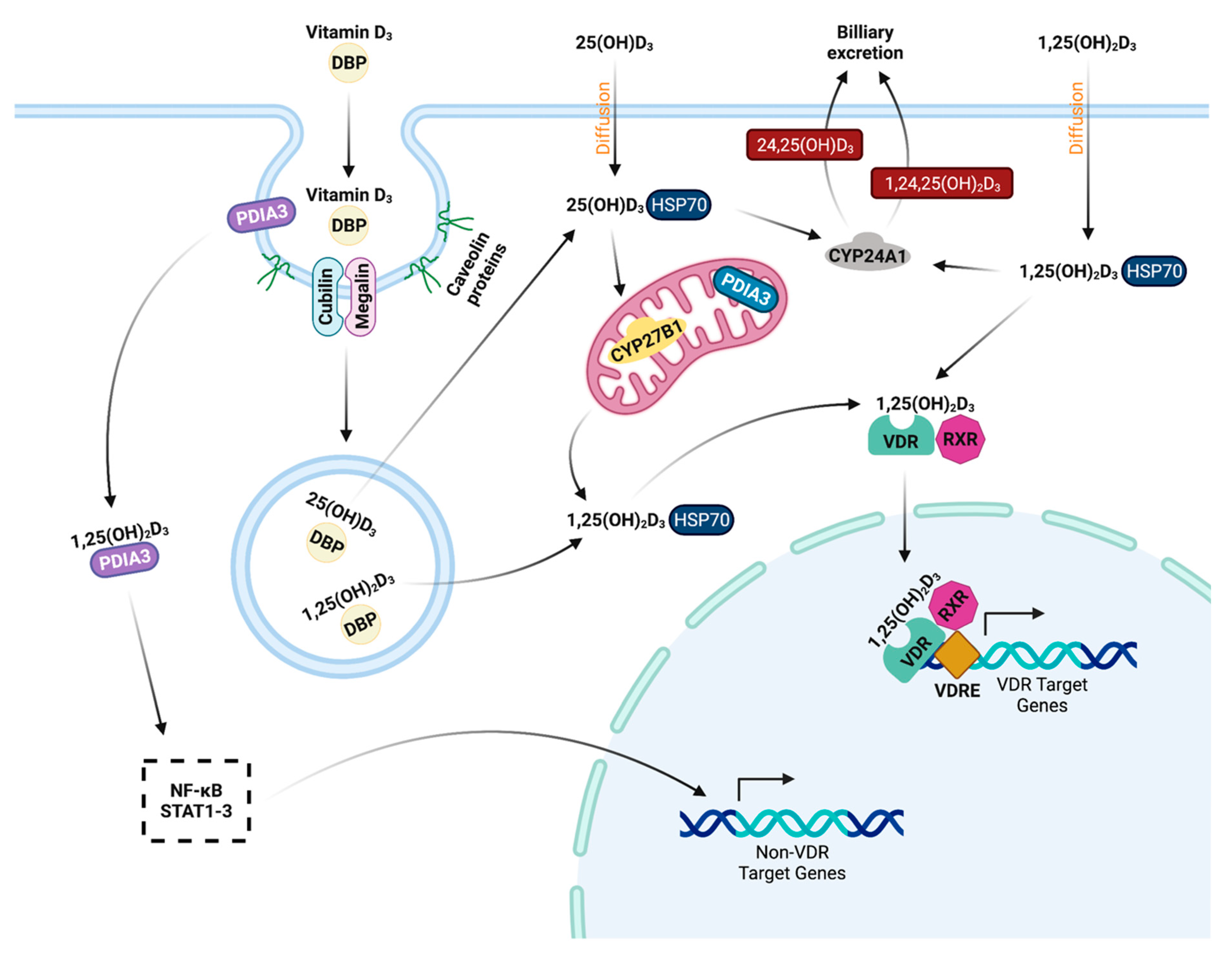
4.3. Classical Function
4.4. Hydroxylase Expression in Immune Cells
4.5. Host Vitamin D Status and Cathelicidins
4.6. Macrophage Phagocytosis and Phenotype
4.7. Cytokines, Nitric Oxide, and β-Defensins
4.8. Macrophage Endosomal Trafficking
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10091865
References
- Buergelt, C.D.; Hall, C.; McEntee, K.; Duncan, J.R. Pathological Evaluation of Paratuberculosis in Naturally Infected Cattle. Vet. Pathol. 1978, 15, 196–207.
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-Level Economic Losses Associated with Johne’s Disease on US Dairy Operations. Prev. Vet. Med. 1999, 40, 179–192.
- Rasmussen, P.; Barkema, H.W.; Mason, S.; Beaulieu, E.; Hall, D.C. Economic Losses Due to Johne’s Disease (Paratuberculosis) in Dairy Cattle. J. Dairy Sci. 2021, 104, 3123–3143.
- Batt, S.M.; Minnikin, D.E.; Besra, G.S. The Thick Waxy Coat of Mycobacteria, a Protective Layer against Antibiotics and the Host’s Immune System. Biochem. J. 2020, 477, 1983–2006.
- Gao, L.-Y.; Laval, F.; Lawson, E.H.; Groger, R.K.; Woodruff, A.; Morisaki, J.H.; Cox, J.S.; Daffe, M.; Brown, E.J. Requirement for kasB in Mycobacterium Mycolic Acid Biosynthesis, Cell Wall Impermeability and Intracellular Survival: Implications for Therapy. Mol. Microbiol. 2003, 49, 1547–1563.
- Salgado, M.; Collins, M.T.; Salazar, F.; Kruze, J.; Bölske, G.; Söderlund, R.; Juste, R.; Sevilla, I.A.; Biet, F.; Troncoso, F.; et al. Fate of Mycobacterium avium Subsp. paratuberculosis after Application of Contaminated Dairy Cattle Manure to Agricultural Soils. Appl. Environ. Microbiol. 2011, 77, 2122–2129.
- Whittington, R.J.; Marshall, D.J.; Nicholls, P.J.; Marsh, I.B.; Reddacliff, L.A. Survival and Dormancy of Mycobacterium avium Subsp. paratuberculosis in the Environment. Appl. Environ. Microbiol. 2004, 70, 2989–3004.
- Whittington, R.J.; Marsh, I.B.; Reddacliff, L.A. Survival of Mycobacterium avium Subsp. paratuberculosis in Dam Water and Sediment. Appl. Environ. Microbiol. 2005, 71, 5304–5308.
- Chiodini, R.J.; Van Kruiningen, H.J. Eastern White-Tailed Deer as a Reservoir of Ruminant Paratuberculosis. J. Am. Vet. Med. Assoc. 1983, 182, 168–169.
- Reyes-García, R.; Pérez-de-la-Lastra, J.M.; Vicente, J.; Ruiz-Fons, F.; Garrido, J.M.; Gortázar, C. Large-Scale ELISA Testing of Spanish Red Deer for Paratuberculosis. Vet. Immunol. Immunopathol. 2008, 124, 75–81.
- Mortier, R.A.R.; Barkema, H.W.; Bystrom, J.M.; Illanes, O.; Orsel, K.; Wolf, R.; Atkins, G.; De Buck, J. Evaluation of Age-Dependent Susceptibility in Calves Infected with Two Doses of Mycobacterium avium Subspecies paratuberculosis Using Pathology and Tissue Culture. Vet. Res. 2013, 44, 94.
- Windsor, P.A.; Whittington, R.J. Evidence for Age Susceptibility of Cattle to Johne’s Disease. Vet. J. 2010, 184, 37–44.
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis Cultured from Milk and Supramammary Lymph Nodes of Infected Asymptomatic Cows. J. Clin. Microbiol. 1992, 30, 166–171.
- Sweeney, R.W.; Whitlock, R.H.; Rosenberger, A.E. Mycobacterium paratuberculosis Isolated from Fetuses of Infected Cows Not Manifesting Signs of the Disease. Am. J. Vet. Res. 1992, 53, 477–480.
- Whittington, R.J.; Windsor, P.A. In Utero Infection of Cattle with Mycobacterium avium Subsp. paratuberculosis: A Critical Review and Meta-Analysis. Vet. J. 2009, 179, 60–69.
- Mitchell, R.M.; Schukken, Y.; Koets, A.; Weber, M.; Bakker, D.; Stabel, J.R.; Whitlock, R.H.; Louzoun, Y. Differences in Intermittent and Continuous Fecal Shedding Patterns between Natural and Experimental Mycobacterium avium Subspecies paratuberculosis Infections in Cattle. Vet. Res. 2015, 46, 66.
- Schukken, Y.H.; Whitlock, R.H.; Wolfgang, D.; Grohn, Y.; Beaver, A.; VanKessel, J.; Zurakowski, M.; Mitchell, R. Longitudinal Data Collection of Mycobacterium avium Subspecies paratuberculosis Infections in Dairy Herds: The Value of Precise Field Data. Vet. Res. 2015, 46, 65.
- Whitlock, R.H.; Buergelt, C. Preclinical and Clinical Manifestations of Paratuberculosis (Including Pathology). Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 345–356.
- Sweeney, R.W. Pathogenesis of Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 537–546.
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13.
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004, 25, 677–686.
- Ambarus, C.A.; Krausz, S.; van Eijk, M.; Hamann, J.; Radstake, T.R.D.J.; Reedquist, K.A.; Tak, P.P.; Baeten, D.L.P. Systematic Validation of Specific Phenotypic Markers for in Vitro Polarized Human Macrophages. J. Immunol. Methods 2012, 375, 196–206.
- Grewal, I.S.; Flavell, R.A. A Central Role of CD40 Ligand in the Regulation of CD4+ T-Cell Responses. Immunol. Today 1996, 17, 410–414.
- Grewal, I.S.; Flavell, R.A. CD40 and CD154 in Cell-Mediated Immunity. Annu. Rev. Immunol. 1998, 16, 111–135.
- Kato, T.; Hakamada, R.; Yamane, H.; Nariuchi, H. Induction of IL-12 P40 Messenger RNA Expression and IL-12 Production of Macrophages via CD40-CD40 Ligand Interaction. J. Immunol. 1996, 156, 3932–3938.
- Sommer, S.; Pudrith, C.B.; Colvin, C.J.; Coussens, P.M. Mycobacterium avium Subspecies paratuberculosis Suppresses Expression of IL-12p40 and iNOS Genes Induced by Signalling through CD40 in Bovine Monocyte-Derived Macrophages. Vet. Immunol. Immunopathol. 2009, 128, 44–52.
- Stabel, J.R. Production of γ-Interferon by Peripheral Blood Mononuclear Cells: An Important Diagnostic Tool for Detection of Subclinical Paratuberculosis. J. Vet. Diagn. Investig. 1996, 8, 345–350.
- Stabel, J.R. Cytokine Secretion by Peripheral Blood Mononuclear Cells from Cows Infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 2000, 61, 754–760.
- Koo, H.C.; Park, Y.H.; Hamilton, M.J.; Barrington, G.M.; Davies, C.J.; Kim, J.B.; Dahl, J.L.; Waters, W.R.; Davis, W.C. Analysis of the Immune Response to Mycobacterium avium Subsp. paratuberculosis in Experimentally Infected Calves. Infect. Immun. 2004, 72, 6870–6883.
- Bonecini-Almeida, M.G.; Chitale, S.; Boutsikakis, I.; Geng, J.; Doo, H.; He, S.; Ho, J.L. Induction of In Vitro Human Macrophage Anti-Mycobacterium tuberculosis Activity: Requirement for IFN-γ and Primed Lymphocytes. J. Immunol. 1998, 160, 4490–4499.
- Hostetter, J.M.; Steadham, E.M.; Haynes, J.S.; Bailey, T.B.; Cheville, N.F. Cytokine Effects on Maturation of the Phagosomes Containing Mycobacteria avium Subspecies paratuberculosis in J774 Cells. FEMS Immunol. Med. Microbiol. 2002, 34, 127–134.
- Lee, B.O.; Haynes, L.; Eaton, S.M.; Swain, S.L.; Randall, T.D. The Biological Outcome of CD40 Signaling Is Dependent on the Duration of CD40 Ligand Expression: Reciprocal Regulation by Interleukin (IL)-4 and IL-12. J. Exp. Med. 2002, 196, 693–704.
- Rogers, P.R.; Croft, M. Peptide Dose, Affinity, and Time of Differentiation Can Contribute to the Th1/Th2 Cytokine Balance. J. Immunol. 1999, 163, 1205–1213.
- Buechler, C.; Ritter, M.; Orsó, E.; Langmann, T.; Klucken, J.; Schmitz, G. Regulation of Scavenger Receptor CD163 Expression in Human Monocytes and Macrophages by Pro- and Antiinflammatory Stimuli. J. Leukoc. Biol. 2000, 67, 97–103.
- Khare, S.; Drake, K.L.; Lawhon, S.D.; Nunes, J.E.S.; Figueiredo, J.F.; Rossetti, C.A.; Gull, T.; Everts, R.E.; Lewin, H.A.; Adams, L.G. Systems Analysis of Early Host Gene Expression Provides Clues for Transient Mycobacterium avium Ssp avium vs. Persistent Mycobacterium avium Ssp paratuberculosis Intestinal Infections. PLoS ONE 2016, 11, e0161946.
- Hussain, T.; Shah, S.Z.A.; Zhao, D.; Sreevatsan, S.; Zhou, X. The Role of IL-10 in Mycobacterium avium Subsp. paratuberculosis. Cell Commun. Signal. 2016, 14, 29.
- Khalifeh, M.S.; Stabel, J.R. Effects of Gamma Interferon, Interleukin-10, and Transforming Growth Factor β on the Survival of Mycobacterium avium Subsp. paratuberculosis in Monocyte-Derived Macrophages from Naturally Infected Cattle. Infect. Immun. 2004, 72, 1974–1982.
- Jenvey, C.J.; Shircliff, A.L.; Bannantine, J.P.; Stabel, J.R. Phenotypes of Macrophages Present in the Intestine Are Impacted by Stage of Disease in Cattle Naturally Infected with Mycobacterium avium Subsp. paratuberculosis. PLoS ONE 2019, 14, e0217649.
- Roussey, J.A.; Steibel, J.P.; Coussens, P.M. Regulatory T Cell Activity and Signs of T Cell Unresponsiveness in Bovine Paratuberculosis. Front. Vet. Sci. 2014, 1, 20.
- Haddad, J.J.; Saadé, N.E.; Safieh-Garabedian, B. Interleukin-10 and the Regulation of Mitogen-Activated Protein Kinases: Are These Signalling Modules Targets for the Anti-Inflammatory Action of This Cytokine? Cell. Signal. 2003, 15, 255–267.
- Weiss, D.J.; Souza, C.D. Review Paper: Modulation of Mononuclear Phagocyte Function by Mycobacterium avium Subsp. paratuberculosis. Vet. Pathol. 2008, 45, 829–841.
- Weiss, D.J.; Souza, C.D.; Evanson, O.A.; Sanders, M.; Rutherford, M. Bovine Monocyte TLR2 Receptors Differentially Regulate the Intracellular Fate of Mycobacterium avium Subsp. paratuberculosis and Mycobacterium avium Subsp. avium. J. Leukoc. Biol. 2008, 83, 48–55.
- de Almeida, D.E.; Colvin, C.J.; Coussens, P.M. Antigen-Specific Regulatory T Cells in Bovine Paratuberculosis. Vet. Immunol. Immunopathol. 2008, 125, 234–245.
- Stabel, J.R.; Bannantine, J.P. Divergent Antigen-Specific Cellular Immune Responses during Asymptomatic Subclinical and Clinical States of Disease in Cows Naturally Infected with Mycobacterium avium Subsp. paratuberculosis. Infect. Immun. 2019, 88, e00650-19.
- Wherry, T.L.T.; Mooyottu, S.; Stabel, J.R. Effects of 1,25-Dihydroxyvitamin D3 and 25-Hydroxyvitamin D3 on PBMCs from Dairy Cattle Naturally Infected with Mycobacterium avium Subsp. paratuberculosis. Front. Vet. Sci. 2022, 9, 830144.
- Alvarez, A.J.; Endsley, J.J.; Werling, D.; Mark Estes, D. WC1 + γδ T Cells Indirectly Regulate Chemokine Production During Mycobacterium bovis Infection in SCID-Bo Mice. Transbound. Emerg. Dis. 2009, 56, 275–284.
- Guzman, E.; Hope, J.; Taylor, G.; Smith, A.L.; Cubillos-Zapata, C.; Charleston, B. Bovine γδ T Cells Are a Major Regulatory T Cell Subset. J. Immunol. Baltim. Md 1950 2014, 193, 208–222.
- Price, S.J.; Hope, J.C. Enhanced Secretion of Interferon-γ by Bovine γδ T Cells Induced by Coculture with Mycobacterium bovis-Infected Dendritic Cells: Evidence for Reciprocal Activating Signals. Immunology 2009, 126, 201–208.
- Albarrak, S.M.; Waters, W.R.; Stabel, J.R.; Hostetter, J.M. WC1+ γδ T Cells from Cattle Naturally Infected with Mycobacterium avium Subsp. paratuberculosis Respond Differentially to Stimulation with PPD-J. Vet. Immunol. Immunopathol. 2017, 190, 57–64.
- Plattner, B.L.; Doyle, R.T.; Hostetter, J.M. Gamma-Delta T Cell Subsets Are Differentially Associated with Granuloma Development and Organization in a Bovine Model of Mycobacterial Disease. Int. J. Exp. Pathol. 2009, 90, 587–597.
- Koets, A.P.; Eda, S.; Sreevatsan, S. The within Host Dynamics of Mycobacterium avium Ssp. paratuberculosis Infection in Cattle: Where Time and Place Matter. Vet. Res. 2015, 46, 61.
- Lei, L.; Plattner, B.L.; Hostetter, J.M. Live Mycobacterium avium Subsp. paratuberculosis and a Killed-Bacterium Vaccine Induce Distinct Subcutaneous Granulomas, with Unique Cellular and Cytokine Profiles. Clin. Vaccine Immunol. 2008, 15, 783–793.
- Da Silva, D.A.A.; da Silva, M.V.; Barros, C.C.O.; Alexandre, P.B.D.; Timóteo, R.P.; Catarino, J.S.; Sales-Campos, H.; Machado, J.R.; Rodrigues, D.B.R.; Oliveira, C.J.; et al. TNF-α Blockade Impairs in Vitro Tuberculous Granuloma Formation and down Modulate Th1, Th17 and Treg Cytokines. PLoS ONE 2018, 13, e0194430.
- Steinbach, S.; Vordermeier, H.M.; Jones, G.J. CD4+ and γδ T Cells Are the Main Producers of IL-22 and IL-17A in Lymphocytes from Mycobacterium bovis-Infected Cattle. Sci. Rep. 2016, 6, 29990.
- Khader, S.A.; Guglani, L.; Rangel-Moreno, J.; Gopal, R.; Junecko, B.A.F.; Fountain, J.J.; Martino, C.; Pearl, J.E.; Tighe, M.; Lin, Y.; et al. IL-23 Is Required for Long-Term Control of Mycobacterium tuberculosis and B Cell Follicle Formation in the Infected Lung. J. Immunol. 2011, 187, 5402–5407.
- DeKuiper, J.L.; Cooperider, H.E.; Lubben, N.; Ancel, C.M.; Coussens, P.M. Mycobacterium avium Subspecies paratuberculosis Drives an Innate Th17-like T Cell Response Regardless of the Presence of Antigen-Presenting Cells. Front. Vet. Sci. 2020, 7, 108.
- Funk, C. On the Chemical Nature of the Substance Which Cures Polyneuritis in Birds Induced by a Diet of Polished Rice. J. Physiol. 1911, 43, 395–400.
- Holst, A.; Frölich, T. Experimental Studies Relating to “Ship-Beri-Beri” and Scurvy. J. Hyg. 1907, 7, 634–671.
- Hopkins, F.G. Feeding Experiments Illustrating the Importance of Accessory Factors in Normal Dietaries. J. Physiol. 1912, 44, 425–460.
- McCollum, E.V.; Simmonds, N.; Becker, J.E.; Shipley, P.G. Studies on Experimental Rickets: XXI. an Experimental Demonstration of the Existence of a Vitamin Which Promotes Calcium Deposition. J. Biol. Chem. 1922, 53, 293–312.
- Windaus, A.; Bock, F. Über Das Provitamin Aus Dem Sterin Der Schweineschwarte. Z. Physiol. Chem. 1937, 245, 168–170.
- Armas, L.A.G.; Hollis, B.W.; Heaney, R.P. Vitamin D2 Is Much Less Effective than Vitamin D3 in Humans. J. Clin. Endocrinol. Metab. 2004, 89, 5387–5391.
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of Vitamin D2 and Vitamin D3 Supplementation in Raising Serum 25-Hydroxyvitamin D Status: A Systematic Review and Meta-Analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364.
- Ponchon, G.; Kennan, A.L.; DeLuca, H.F. “Activation” of Vitamin D by the Liver. J. Clin. Investig. 1969, 48, 2032–2037.
- Prosser, D.E.; Jones, G. Enzymes Involved in the Activation and Inactivation of Vitamin D. Trends Biochem. Sci. 2004, 29, 664–673.
- Pacheco, H.A.; da Silva, S.; Sigdel, A.; Mak, C.K.; Galvão, K.N.; Texeira, R.A.; Dias, L.T.; Peñagaricano, F. Gene Mapping and Gene-Set Analysis for Milk Fever Incidence in Holstein Dairy Cattle. Front. Genet. 2018, 9, 465.
- Reinhardt, T.A.; Horst, R.L.; Goff, J.P. Calcium, Phosphorus, and Magnesium Homeostasis in Ruminants. Vet. Clin. N. Am. Food Anim. Pract. 1988, 4, 331–350.
- Takeyama, K.; Kitanaka, S.; Sato, T.; Kobori, M.; Yanagisawa, J.; Kato, S. 25-Hydroxyvitamin D3 1α-Hydroxylase and Vitamin D Synthesis. Science 1997, 277, 1827–1830.
- Fraser, D.R.; Kodicek, E. Unique Biosynthesis by Kidney of a Biologically Active Vitamin D Metabolite. Nature 1970, 228, 764–766.
- Paulson, S.K.; DeLuca, H.F. Subcellular Location and Properties of Rat Renal 25-Hydroxyvitamin D3-1α-Hydroxylase. J. Biol. Chem. 1985, 260, 11488–11492.
- Weisman, Y.; Harell, A.; Edelstein, S.; David, M.; Spirer, Z.; Golander, A. 1α, 25-Dihydroxyvitamin D3, and 24,25-Dihydroxyvitamin D3 in Vitro Synthesis by Human Decidua and Placenta. Nature 1979, 281, 317–319.
- Howard, G.A.; Turner, R.T.; Sherrard, D.J.; Baylink, D.J. Human Bone Cells in Culture Metabolize 25-Hydroxyvitamin D3 to 1,25-Dihydroxyvitamin D3 and 24,25-Dihydroxyvitamin D3. J. Biol. Chem. 1981, 256, 7738–7740.
- Turner, R.T.; Puzas, J.E.; Forte, M.D.; Lester, G.E.; Gray, T.K.; Howard, G.A.; Baylink, D.J. In Vitro Synthesis of 1α,25-Dihydroxycholecalciferol and 24,25-Dihydroxycholecalciferol by Isolated Calvarial Cells. Proc. Natl. Acad. Sci. USA 1980, 77, 5720–5724.
- Reichel, H.; Koeffler, H.P.; Norman, A.W. Synthesis in Vitro of 1,25-Dihydroxyvitamin D3 and 24,25-Dihydroxyvitamin D3 by Interferon-γ-Stimulated Normal Human Bone Marrow and Alveolar Macrophages. J. Biol. Chem. 1987, 262, 10931–10937.
- Bikle, D.D.; Nemanic, M.K.; Whitney, J.O.; Elias, P.W. Neonatal Human Foreskin Keratinocytes Produce 1,25-Dihydroxyvitamin D3. Biochemistry 1986, 25, 1545–1548.
- Bikle, D.D.; Nemanic, M.K.; Gee, E.; Elias, P. 1,25-Dihydroxyvitamin D3 Production by Human Keratinocytes. Kinetics and Regulation. J. Clin. Investig. 1986, 78, 557–566.
- Hollis, B.W.; Wagner, C.L.; Drezner, M.K.; Binkley, N.C. Circulating Vitamin D3 and 25-Hydroxyvitamin D in Humans: An Important Tool to Define Adequate Nutritional Vitamin D Status. J. Steroid Biochem. Mol. Biol. 2007, 103, 631–634.
- Jones, G. Pharmacokinetics of Vitamin D Toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S.
- Horst, R.L.; Littledike, E.T.; Riley, J.L.; Napoli, J.L. Quantitation of Vitamin D and Its Metabolites and Their Plasma Concentrations in Five Species of Animals. Anal. Biochem. 1981, 116, 189–203.
- Antoniucci, D.M.; Black, D.M.; Sellmeyer, D.E. Serum 25-Hydroxyvitamin D Is Unaffected by Multiple Freeze-Thaw Cycles. Clin. Chem. 2005, 51, 258–261.
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the Free Fraction of 25-Hydroxyvitamin D in Serum and Its Regulation by Albumin and the Vitamin D-Binding Protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959.
- Hollis, B.W. Comparison of Equilibrium and Disequilibrium Assay Conditions for Ergocalciferol, Cholecalciferol and Their Major Metabolites. J. Steroid Biochem. 1984, 21, 81–86.
- Hoy, D.A.; Ramberg, C.F., Jr.; Horst, R.L. Evidence That Discrimination against Ergocalciferol by the Chick Is the Result of Enhanced Metabolic Clearance Rates for Its Mono- and Dihydroxylated Metabolites. J. Nutr. 1988, 118, 633–638.
- Chun, R.F.; Lauridsen, A.L.; Suon, L.; Zella, L.A.; Pike, J.W.; Modlin, R.L.; Martineau, A.R.; Wilkinson, R.J.; Adams, J.; Hewison, M. Vitamin D-Binding Protein Directs Monocyte Responses to 25-Hydroxy- and 1,25-Dihydroxyvitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 3368–3376.
- Vanham, G.; Van Baelen, H.; Tan, B.K.; Bouillon, R. The Effect of Vitamin D Analogs and of Vitamin D-Binding Protein on Lymphocyte Proliferation. J. Steroid Biochem. 1988, 29, 381–386.
- Vargas, S.; Bouillon, R.; Van Baelen, H.; Raisz, L.G. Effects of Vitamin D-Binding Protein on Bone Resorption Stimulated by 1,25 Dihydroxyvitamin D3. Calcif. Tissue Int. 1990, 47, 164–168.
- Adams, J.S.; Chen, H.; Chun, R.; Ren, S.; Wu, S.; Gacad, M.; Nguyen, L.; Ride, J.; Liu, P.; Modlin, R.; et al. Substrate and Enzyme Trafficking as a Means of Regulating 1,25-Dihydroxyvitamin D Synthesis and Action: The Human Innate Immune Response. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22 (Suppl. S2), V20–V24.
- Sirajudeen, S.; Shah, I.; Al Menhali, A. A Narrative Role of Vitamin D and Its Receptor: With Current Evidence on the Gastric Tissues. Int. J. Mol. Sci. 2019, 20, 3832.
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular Mechanisms of Vitamin D Action. Calcif. Tissue Int. 2013, 92, 77–98.
- Zmijewski, M.A.; Carlberg, C. Vitamin D Receptor(s): In the Nucleus but Also at Membranes? Exp. Dermatol. 2020, 29, 876–884.
- Chen, J.; Doroudi, M.; Cheung, J.; Grozier, A.L.; Schwartz, Z.; Boyan, B.D. Plasma Membrane Pdia3 and VDR Interact to Elicit Rapid Responses to 1α,25(OH)2D3. Cell. Signal. 2013, 25, 2362–2373.
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 Activates the Autolysosomal Degradation Function against Helicobacter Pylori through the PDIA3 Receptor in Gastric Epithelial Cells. Autophagy 2019, 15, 707–725.
- Wu, W.; Beilhartz, G.; Roy, Y.; Richard, C.L.; Curtin, M.; Brown, L.; Cadieux, D.; Coppolino, M.; Farach-Carson, M.C.; Nemere, I.; et al. Nuclear Translocation of the 1,25D3-MARRS (Membrane Associated Rapid Response to Steroids) Receptor Protein and NFκB in Differentiating NB4 Leukemia Cells. Exp. Cell Res. 2010, 316, 1101–1108.
- Siu-Caldera, M.L.; Zou, L.; Ehrlich, M.G.; Schwartz, E.R.; Ishizuka, S.; Reddy, G.S. Human Osteoblasts in Culture Metabolize Both 1α,25-Dihydroxyvitamin D3 and Its Precursor 25-Hydroxyvitamin D3 into Their Respective Lactones. Endocrinology 1995, 136, 4195–4203.
- Clements, M.R.; Chalmers, T.M.; Fraser, D.R. Enterohepatic Circulation of Vitamin D: A Reappraisal of the Hypothesis. Lancet 1984, 323, 1376–1379.
- Hahn, T.J.; Birge, S.J.; Scharp, C.R.; Avioli, L.V. Phenobarbital-Induced Alterations in Vitamin D Metabolism. J. Clin. Investig. 1972, 51, 741–748.
- Horst, R.L.; Goff, J.P.; Reinhardt, T.A. Calcium and Vitamin D Metabolism in the Dairy Cow. J. Dairy Sci. 1994, 77, 1936–1951.
- Horst, R.L.; Reinhardt, T.A.; Reddy, G.S. CHAPTER 2—Vitamin D Metabolism. In Vitamin D (Second Edition); Feldman, D., Ed.; Academic Press: Burlington, VT, USA, 2005; pp. 15–36. ISBN 978-0-12-252687-9.
- Morgan, J.W.; Reddy, G.S.; Uskokovic, M.R.; May, B.K.; Omdahl, J.L.; Maizel, A.L.; Sharma, S. Functional Block for 1α,25-Dihydroxyvitamin D3-Mediated Gene Regulation in Human B Lymphocytes. J. Biol. Chem. 1994, 269, 13437–13443.
- St-Arnaud, R.; Arabian, A.; Travers, R.; Barletta, F.; Raval-Pandya, M.; Chapin, K.; Depovere, J.; Mathieu, C.; Christakos, S.; Demay, M.B.; et al. Deficient Mineralization of Intramembranous Bone in Vitamin D-24-Hydroxylase-Ablated Mice Is Due to Elevated 1,25-Dihydroxyvitamin D and Not to the Absence of 24,25-Dihydroxyvitamin D. Endocrinology 2000, 141, 2658–2666.
- Cutolo, M.; Plebani, M.; Shoenfeld, Y.; Adorini, L.; Tincani, A. Chapter Fourteen—Vitamin D Endocrine System and the Immune Response in Rheumatic Diseases. In Vitamins & Hormones; Litwack, G., Ed.; Vitamins and the Immune System; Academic Press: Cambridge, MA, USA, 2011; Volume 86, pp. 327–351.
- Beck, G.R.; Zerler, B.; Moran, E. Phosphate Is a Specific Signal for Induction of Osteopontin Gene Expression. Proc. Natl. Acad. Sci. USA 2000, 97, 8352–8357.
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95.
- Conigrave, A.D. The Calcium-Sensing Receptor and the Parathyroid: Past, Present, Future. Front. Physiol. 2016, 7, 563.
- Adams, J.S.; Sharma, O.P.; Gacad, M.A.; Singer, F.R. Metabolism of 25-Hydroxyvitamin D3 by Cultured Pulmonary Alveolar Macrophages in Sarcoidosis. J. Clin. Investig. 1983, 72, 1856–1860.
- Insogna, K.L.; Dreyer, B.E.; Mitnick, M.; Ellison, A.F.; Broadus, A.E. Enhanced Production Rate of 1,25-Dihydroxyvitamin D in Sarcoidosis. J. Clin. Endocrinol. Metab. 1988, 66, 72–75.
- Gyetko, M.R.; Hsu, C.H.; Wilkinson, C.C.; Patel, S.; Young, E. Monocyte 1α-Hydroxylase Regulation: Induction by Inflammatory Cytokines and Suppression by Dexamethasone and Uremia Toxin. J. Leukoc. Biol. 1993, 54, 17–22.
- Noyola-Martínez, N.; Díaz, L.; Zaga-Clavellina, V.; Avila, E.; Halhali, A.; Larrea, F.; Barrera, D. Regulation of CYP27B1 and CYP24A1 Gene Expression by Recombinant Pro-Inflammatory Cytokines in Cultured Human Trophoblasts. J. Steroid Biochem. Mol. Biol. 2014, 144, 106–109.
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune Regulation of 25-Hydroxyvitamin-D3-1α-Hydroxylase in Human Monocytes. J. Bone Miner. Res. 2006, 21, 37–47.
- Nelson, C.D.; Reinhardt, T.A.; Thacker, T.C.; Beitz, D.C.; Lippolis, J.D. Modulation of the Bovine Innate Immune Response by Production of 1α,25-Dihydroxyvitamin D3 in Bovine Monocytes. J. Dairy Sci. 2010, 93, 1041–1049.
- Wherry, T.L.T.; Dassanayake, R.P.; Casas, E.; Mooyottu, S.; Bannantine, J.P.; Stabel, J.R. Exogenous Vitamin D3 Modulates Response of Bovine Macrophages to Mycobacterium avium Subsp. paratuberculosis Infection and Is Dependent Upon Stage of Johne’s Disease. Front. Cell. Infect. Microbiol. 2022, 11, 1446.
- Fabri, M.; Stenger, S.; Shin, D.-M.; Yuk, J.-M.; Liu, P.T.; Realegeno, S.; Lee, H.-M.; Krutzik, S.R.; Schenk, M.; Sieling, P.A.; et al. Vitamin D Is Required for IFN-γ–Mediated Antimicrobial Activity of Human Macrophages. Sci. Transl. Med. 2011, 3, 104ra102.
- Stabel, J.R.; Reinhardt, T.A.; Hempel, R.J. Short Communication: Vitamin D Status and Responses in Dairy Cows Naturally Infected with Mycobacterium avium Ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 1594–1600.
- Nelson, C.D.; Reinhardt, T.A.; Beitz, D.C.; Lippolis, J.D. In Vivo Activation of the Intracrine Vitamin D Pathway in Innate Immune Cells and Mammary Tissue during a Bacterial Infection. PLoS ONE 2010, 5, e15469.
- Merriman, K.E.; Kweh, M.F.; Powell, J.L.; Lippolis, J.D.; Nelson, C.D. Multiple β-Defensin Genes Are Upregulated by the Vitamin D Pathway in Cattle. J. Steroid Biochem. Mol. Biol. 2015, 154, 120–129.
- Nelson, C.D.; Nonnecke, B.J.; Reinhardt, T.A.; Waters, W.R.; Beitz, D.C.; Lippolis, J.D. Regulation of Mycobacterium-Specific Mononuclear Cell Responses by 25-Hydroxyvitamin D3. PLoS ONE 2011, 6, e21674.
- Merriman, K.E.; Poindexter, M.B.; Kweh, M.F.; Santos, J.E.P.; Nelson, C.D. Intramammary 1,25-Dihydroxyvitamin D3 Treatment Increases Expression of Host-Defense Genes in Mammary Immune Cells of Lactating Dairy Cattle. J. Steroid Biochem. Mol. Biol. 2017, 173, 33–41.
- Wherry, T.L.T.; Heggen, M.; Shircliff, A.L.; Mooyottu, S.; Stabel, J.R. Stage of Infection with Mycobacterium avium Subsp. paratuberculosis Impacts Expression of Rab5, Rab7, and CYP27B1 in Macrophages Within the Ileum of Naturally Infected Cows. Vet. Microbiol. 2022. manuscript in review.
- Adams, J.S.; Hewison, M. Update in Vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478.
- Hollis, B.W. Circulating 25-Hydroxyvitamin D Levels Indicative of Vitamin D Sufficiency: Implications for Establishing a New Effective Dietary Intake Recommendation for Vitamin D. J. Nutr. 2005, 135, 317–322.
- Nelson, C.D.; Reinhardt, T.A.; Lippolis, J.D.; Sacco, R.E.; Nonnecke, B.J. Vitamin D Signaling in the Bovine Immune System: A Model for Understanding Human Vitamin D Requirements. Nutrients 2012, 4, 181–196.
- Vieth, R. Why the Minimum Desirable Serum 25-Hydroxyvitamin D Level Should Be 75 Nmol/L (30 Ng/Ml). Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 681–691.
- Nelson, C.D.; Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.E.; Powell, J.L.; Drewnoski, M.E.; O’Neil, M.; Beitz, D.C.; Weiss, W.P. Vitamin D Status of Dairy Cattle: Outcomes of Current Practices in the Dairy Industry. J. Dairy Sci. 2016, 99, 10150–10160.
- Nelson, C.D.; Powell, J.L.; Price, D.M.; Hersom, M.J.; Yelich, J.V.; Drewnoski, M.E.; Bird, S.L.; Bridges, G.A. Assessment of Serum 25-Hydroxyvitamin D Concentrations of Beef Cows and Calves across Seasons and Geographical Locations. J. Anim. Sci. 2016, 94, 3958–3965.
- Adams, J.S.; Ren, S.; Liu, P.T.; Chun, R.F.; Lagishetty, V.; Gombart, A.F.; Borregaard, N.; Modlin, R.L.; Hewison, M. Vitamin D-Directed Rheostatic Regulation of Monocyte Antibacterial Responses. J. Immunol. Baltim. Md 1950 2009, 182, 4289–4295.
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting Edge: Vitamin D-Mediated Human Antimicrobial Activity against Mycobacterium tuberculosis Is Dependent on the Induction of Cathelicidin. J. Immunol. 2007, 179, 2060–2063.
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E.W. The Human Antimicrobial Peptide LL-37 Is a Multifunctional Modulator of Innate Immune Responses. J. Immunol. 2002, 169, 3883–3891.
- Wan, M.; van der Does, A.M.; Tang, X.; Lindbom, L.; Agerberth, B.; Haeggström, J.Z. Antimicrobial Peptide LL-37 Promotes Bacterial Phagocytosis by Human Macrophages. J. Leukoc. Biol. 2014, 95, 971–981.
- Scocchi, M.; Wang, S.; Zanetti, M. Structural Organization of the Bovine Cathelicidin Gene Family and Identification of a Novel Member. FEBS Lett. 1997, 417, 311–315.
- Whelehan, C.J.; Barry-Reidy, A.; Meade, K.G.; Eckersall, P.; Chapwanya, A.; Narciandi, F.; Lloyd, A.T.; O’Farrelly, C. Characterisation and Expression Profile of the Bovine Cathelicidin Gene Repertoire in Mammary Tissue. BMC Genom. 2014, 15, 128.
- Elsik, C.G.; Tellam, R.L.; Worley, K.C. The Genome Sequence of Taurine Cattle: A Window to Ruminant Biology and Evolution. Science 2009, 324, 522–528.
- Patil, A.A.; Cai, Y.; Sang, Y.; Blecha, F.; Zhang, G. Cross-Species Analysis of the Mammalian β-Defensin Gene Family: Presence of Syntenic Gene Clusters and Preferential Expression in the Male Reproductive Tract. Physiol. Genom. 2005, 23, 5–17.
- García-Barragán, Á.; Gutiérrez-Pabello, J.A.; Alfonseca-Silva, E. Calcitriol Increases Nitric Oxide Production and Modulates Microbicidal Capacity against Mycobacterium bovis in Bovine Macrophages. Comp. Immunol. Microbiol. Infect. Dis. 2018, 59, 17–23.
- Xu, H.; Soruri, A.; Gieseler, R.K.H.; Peters, J.H. 1,25-Dihydroxyvitamin D3 Exerts Opposing Effects to IL-4 on MHC Class-II Antigen Expression, Accessory Activity, and Phagocytosis of Human Monocytes. Scand. J. Immunol. 1993, 38, 535–540.
- Chandra, G.; Selvaraj, P.; Jawahar, M.S.; Banurekha, V.V.; Narayanan, P.R. Effect of Vitamin D3 on Phagocytic Potential of Macrophages with Live Mycobacterium tuberculosis and Lymphoproliferative Response in Pulmonary Tuberculosis. J. Clin. Immunol. 2004, 24, 249–257.
- Small, A.G.; Harvey, S.; Kaur, J.; Putty, T.; Quach, A.; Munawara, U.; Perveen, K.; McPhee, A.; Hii, C.S.; Ferrante, A. Vitamin D Upregulates the Macrophage Complement Receptor Immunoglobulin in Innate Immunity to Microbial Pathogens. Commun. Biol. 2021, 4, 401.
- Zeng, Z.; Surewaard, B.G.J.; Wong, C.H.Y.; Geoghegan, J.A.; Jenne, C.N.; Kubes, P. CRIg Functions as a Macrophage Pattern Recognition Receptor to Directly Bind and Capture Blood-Borne Gram-Positive Bacteria. Cell Host Microbe 2016, 20, 99–106.
- Kim, E.W.; Teles, R.M.B.; Haile, S.; Liu, P.T.; Modlin, R.L. Vitamin D Status Contributes to the Antimicrobial Activity of Macrophages against Mycobacterium leprae. PLoS Negl. Trop. Dis. 2018, 12, e0006608.
- Leidi, M.; Gotti, E.; Bologna, L.; Miranda, E.; Rimoldi, M.; Sica, A.; Roncalli, M.; Palumbo, G.A.; Introna, M.; Golay, J. M2 Macrophages Phagocytose Rituximab-Opsonized Leukemic Targets More Efficiently than M1 Cells In Vitro. J. Immunol. 2009, 182, 4415–4422.
- Wherry, T.L.T.; Dassanayake, R.P.; Bannantine, J.P.; Mooyottu, S.; Stabel, J.R. Vitamin D3 Alters Macrophage Phenotype and Endosomal Trafficking Markers in Dairy Cattle Naturally Infected with Mycobacterium avium Subsp. paratuberculosis. Front. Cell. Infect. Microbiol. 2022. manuscript in review.
- Waters, W.R.; Nonnecke, B.J.; Rahner, T.E.; Palmer, M.V.; Whipple, D.L.; Horst, R.L. Modulation of Mycobacterium bovis-Specific Responses of Bovine Peripheral Blood Mononuclear Cells by 1,25-Dihydroxyvitamin D3. Clin. Diagn. Lab. Immunol. 2001, 8, 1204–1212.
- Nonnecke, B.J.; Waters, W.R.; Foote, M.R.; Horst, R.L.; Fowler, M.A.; Miller, B.L. In Vitro Effects of 1,25-Dihydroxyvitamin D3 on Interferon-γ and Tumor Necrosis Factor-α Secretion by Blood Leukocytes from Young and Adult Cattle Vaccinated with Mycobacterium bovis BCG. Int. J. Vitam. Nutr. Res. 2003, 73, 235–244.
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science 2006, 311, 1770–1773.
- Crowle, A.J.; Ross, E.J.; May, M.H. Inhibition by 1,25(OH)2-Vitamin D3 of the Multiplication of Virulent Tubercle Bacilli in Cultured Human Macrophages. Infect. Immun. 1987, 55, 2945–2950.
- Rook, G.A.; Steele, J.; Fraher, L.; Barker, S.; Karmali, R.; O’Riordan, J.; Stanford, J. Vitamin D3, Gamma Interferon, and Control of Proliferation of Mycobacterium tuberculosis by Human Monocytes. Immunology 1986, 57, 159–163.
- Waters, W.R.; Palmer, M.V.; Nonnecke, B.J.; Whipple, D.L.; Horst, R.L. Mycobacterium bovis Infection of Vitamin D-Deficient NOS2−/− Mice. Microb. Pathog. 2004, 36, 11–17.
- Martinez, N.; Rodney, R.M.; Block, E.; Hernandez, L.L.; Nelson, C.D.; Lean, I.J.; Santos, J.E.P. Effects of Prepartum Dietary Cation-Anion Difference and Source of Vitamin D in Dairy Cows: Health and Reproductive Responses. J. Dairy Sci. 2018, 101, 2563–2578.
- Kweh, M.F.; Merriman, K.E.; Wells, T.L.; Nelson, C.D. Vitamin D Signaling Increases Nitric Oxide and Antioxidant Defenses of Bovine Monocytes. JDS Commun. 2021, 2, 73–79.
- Merriman, K.E.; Powell, J.L.; Santos, J.E.P.; Nelson, C.D. Intramammary 25-Hydroxyvitamin D3 Treatment Modulates Innate Immune Responses to Endotoxin-Induced Mastitis. J. Dairy Sci. 2018, 101, 7593–7607.
- Lippolis, J.D.; Reinhardt, T.A.; Sacco, R.A.; Nonnecke, B.J.; Nelson, C.D. Treatment of an Intramammary Bacterial Infection with 25-Hydroxyvitamin D3. PLoS ONE 2011, 6, e25479.
