Calciotropic hormones, parathyroid hormone (PTH) and calcitonin are involved in the regulation of bone mineral metabolism and maintenance of calcium and phosphate homeostasis in the body. Therefore, an understanding of environmental and genetic factors influencing PTH and calcitonin levels is crucial. Genetic factors are estimated to account for 60% of variations in PTH levels.
- environmental factors
- PTH
- calcitonin
- lifestyle
1. Introduction
Maintenance of calcium homeostasis in the body is crucial since calcium regulates various physiological processes, including cellular signaling, protein and enzyme function, neurotransmission, contractility of the muscles, and blood coagulation [1]. Calcium homeostasis is regulated by parathyroid hormone (PTH), calcitonin, the active form of vitamin D (1α,25-dihydroxyvitamin D (1,25(OH)2D3)), and serum calcium and phosphate levels. Regulation of phosphate metabolism is also important as phosphate is involved in protein and enzyme function, cell signaling, and skeletal mineralization and is a component of cell membranes and nucleic acids [2][3]. The main factors that regulate phosphate homeostasis are PTH, fibroblast growth factor 23 (FGF-23), 1,25(OH)2D3, and Klotho [3]. Calcitonin is also involved in the regulation of phosphate levels [4][5]. PTH is released from the parathyroid glands [6], while calcitonin is released from thyroid C-cells [7]. Alternation of PTH levels can lead to the development of hyperparathyroidism and hypoparathyroidism. Changes in calcitonin levels have also been observed in pathological conditions (such as medullary thyroid carcinoma [8]). Therefore, variations in PTH and calcitonin levels may indicate that the normal functioning of parathyroid glands and thyroid is altered. Various factors can affect PTH and calcitonin levels, such as genetic factors [9][10][11], demographic factors (age [12][13][14], sex [15][16][17]), and environmental factors [18][19][20][21]. It is estimated that genetic factors account for 60% of variations in PTH levels [9], while the amount to which genetic factors contribute to interindividual variation in calcitonin levels has not been studied (Figure 1).
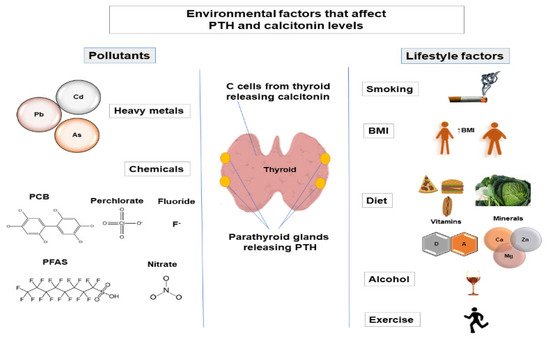
2. Environmental Factors That Affect PTH and Calcitonin Levels
2.1. Lifestyle Factors
2.1.1. Smoking
A lot of studies have investigated the impact of smoking on PTH levels. Most of them reported a decrease in PTH levels in smokers [22][23][24]. Studies investigating the effect of smoking on calcitonin levels have found an increase in calcitonin levels in smokers [17][25][26].
2.1.2. Body Mass Index
Many studies have investigated the influence of body mass index (BMI) on PTH levels. Most of them have shown that an increase in BMI is accompanied by an increase in PTH levels [22][23][27]. However, a study by Yuan et al., showed a positive correlation between BMI and PTH levels in subjects with lower PTH levels (below 65.8 pg/mL), while a negative correlation was observed between BMI and PTH levels in the group of patients with high PTH levels (above 147 pg/mL) [28]. Several studies have investigated the association between BMI and calcitonin levels, reporting conflicting results [17][25].
2.1.3. Diet
Different types of food can affect the level of PTH in the body. A diet high in phosphorus and low in calcium has been shown to increase PTH levels [29][30]. This is logical because both high serum phosphate levels and low serum calcium levels are signals to increase PTH release [31]. Because vitamin D and PTH act together, several studies have tested the effect of vitamin D on PTH levels. About 95% of vitamin D is synthesized in the skin after exposure to sunlight, while 5% of vitamin D comes from food [32]. Since PTH and the active form of vitamin D (1,25(OH)2D) are in an inverse relationship, it is not surprising that most of the studies have reported a decrease in PTH levels after vitamin D intake [33][34][35]. No changes in calcitonin levels were observed after vitamin D intake [36]. Calcium intake has been shown to decrease PTH levels [27][37], which is to be expected since PTH is released in hypocalcemia.
2.1.4. Alcohol
Studies investigating the influence of alcohol on PTH levels have yielded conflicting results. Some studies have found a decrease in PTH levels in alcoholics, while most studies have not reported a significant change in PTH levels due to alcohol consumption [24][38]. Conflicting results were published regarding calcitonin levels in alcoholics, with calcitonin levels being increased, decreased, or unchanged in alcoholics [39][40][41].
2.1.5. Exercise
Studies that have investigated the influence of exercise on PTH levels have reported an increase in PTH levels during and after exercise [20][42][43]. Calcitonin levels increased [44] or did not change [20] during exercise.
2.2. Pollutants
2.2.1. Heavy Metals
Various heavy metals, such as cadmium (Cd), arsenic (As), and lead (Pb), affect PTH levels. It has been shown that PTH levels decrease after cadmium exposure [18][45]. Arsenic exposure did not affect PTH levels [46]. Otherwise, an increase in PTH levels was observed in subjects exposed to lead [47][48].
2.2.2. Chemicals
Only a few studies have investigated the effect of chemicals on PTH levels in humans. Exposure to persistent organochlorine compounds (p,p′-diphenyldichloroethene (p,p′-DDE) and polychlorinated biphenyls (PCBs)) did not affect PTH levels [49][50]. Exposure to perfluoroalkyl substances (PFAS) led to an increase in PTH levels [51]. Di Nisio et al. suggested that perfluoro-octanoic acid (PFOA) binds to vitamin D receptors, causing reduced 1,25(OH)D activity, which in turn increases PTH levels [51]. Fluoride exposure increases PTH levels [52]. According to researchers, excess fluoride alters calcium metabolism and potentially leads to secondary hyperparathyroidism (reviewed in [53]). Exposure to perchlorate, thiocyanate, and nitrate has led to a decrease in PTH levels, but the underlying mechanism of this action is not yet clear [19].
3. Conclusions
Environmental factors affect the levels of PTH and calcitonin, two hormones that regulate calcium and phosphate homeostasis. In terms of lifestyle factors, most studies have shown a decrease in PTH levels in smokers, a positive correlation between BMI and PTH, an increase in PTH levels during exercise, and a decrease in PTH levels after vitamin D and calcium intake. The results of studies on the impact of alcohol consumption and intake of different types of food and micronutrients (except for vitamin D and calcium) showed great variability. Regarding studies that analyzed the effect of pollutants on PTH levels, the clearest relationship was between PTH and cadmium, with PTH levels decreasing after cadmium exposure. While arsenic exposure did not affect PTH levels, lead exposure resulted in increased PTH levels. Several studies have investigated the influence of chemicals on PTH levels in humans. Moreover, data on the effect of chemicals and heavy metals on calcitonin levels in humans are scarce, and most of the knowledge, to date, relies on studies in experimental animals. As for the relationship between lifestyle factors and calcitonin, several studies have been conducted on humans and have given great variability in results. The most consistent results were related to smoking (an increase in calcitonin levels was observed in smokers). Given the important role that PTH and calcitonin play in maintaining calcium and phosphate homeostasis in the body, additional studies on the influence of environmental and genetic factors that could affect the levels of these two hormones are extremely important.
Abbreviations
Funding
This entry is adapted from the peer-reviewed paper 10.3390/ijms23010044
References
- Tebben, P.J.; Kumar, R. The hormonal regulation of calcium metabolism. In Seldin and Geibisch’s The Kidney; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 2, pp. 2249–2272. ISBN 9780123814623.
- Choi, N.W. Kidney and phosphate metabolism. Electrolytes Blood Press. 2008, 6, 77–85, https://doi.org/10.5049/EBP.2008.6.2.77.
- Gattineni, J.; Friedman, P.A. Regulation of hormone-sensitive renal phosphate transport. Horm. 2015, 98, 249–306, https://doi.org/10.1016/BS.VH.2015.01.002.
- Talmage, R.V.; Vanderwiel, C.J.; Matthews, J.L. Calcitonin and phosphate. Cell. Endocrinol. 1981, 24, 235–251, https://doi.org/10.1016/0303-7207(81)90001-0.
- Jafari, N.; Abdollahpour, H.; Falahatkar, B. Stimulatory effects of short-term calcitonin administration on plasma calcium, magnesium, phosphate, and glucose in juvenile Siberian sturgeon Acipenser baerii. Fish Physiol. Biochem. 2020, 46, 1443–1449, https://doi.org/10.1007/S10695-020-00801-Z/FIGURES/1.
- Lofrese, J.J.; Basit, H.; Lappin, S.L. Physiology, Parathyroid; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021.
- Pearse, A.G. The cytochemistry of the thyroid C cells and their relationship to calcitonin. R. Soc. Lond. Ser. B Biol. Sci. 1966, 164, 478–487, https://doi.org/10.1098/RSPB.1966.0044.
- Bae, Y.J.; Schaab, M.; Kratzsch, J. Calcitonin as biomarker for the medullary thyroid carcinoma. Recent Res. Cancer Res. 2015, 204, 117–137, https://doi.org/10.1007/978-3-319-22542-5_5.
- Hunter, D.; De Lange, M.; Snieder, H.; MacGregor, A.J.; Swaminathan, R.; Thakker, R.V.; Spector, T.D. Genetic contribution to bone metabolism, calcium excretion, and vitamin D and parathyroid hormone regulation. Bone Miner. Res. 2001, 16, 371–378, https://doi.org/10.1359/JBMR.2001.16.2.371.
- Robinson-Cohen, C.; Lutsey, P.L.; Kleber, M.E.; Nielson, C.M.; Mitchell, B.D.; Bis, J.C.; Eny, K.M.; Portas, L.; Eriksson, J.; Lorentzon, M.; et al. Genetic variants associated with circulating parathyroid hormone. Am. Soc. Nephrol. 2017, 28, 1553–1565, https://doi.org/10.1681/ASN.2016010069.
- Matana, A.; Brdar, D.; Torlak, V.; Boutin, T.; Popović, M.; Gunjača, I.; Kolčić, I.; Boraska Perica, V.; Punda, A.; Polašek, O.; et al. Genome-wide meta-analysis identifies novel loci associated with parathyroid hormone level. Med. 2018, 24, 15, https://doi.org/10.1186/S10020-018-0018-5.
- Deftos, L.J.; Weisman, M.H.; Williams, G.W.; Karpf, D.B.; Frumar, A.M.; Davidson, B.J.; Parthemore, J.G.; Judd, H.L. Influence of age and sex on plasma calcitonin in human beings. Engl. J. Med. 1980, 302, 1351–1353, https://doi.org/10.1056/NEJM198006123022407.
- Haden, S.T.; Brown, E.M.; Hurwitz, S.; Scott, J.; Fuleihan, G.E.H. The effects of age and gender on parathyroid hormone dynamics. Endocrinol. 2000, 52, 329–338, https://doi.org/10.1046/J.1365-2265.2000.00912.X.
- Carrivick, S.J.; Walsh, J.P.; Brown, S.J.; Wardrop, R.; Hadlow, N.C. Brief report: Does PTH increase with age, independent of 25-hydroxyvitamin D, phosphate, renal function, and ionized calcium? Clin. Endocrinol. Metab. 2015, 100, 2131–2134, https://doi.org/10.1210/JC.2014-4370.
- Tiegs, R.D.; Body, J.J.; Barta, J.M.; Heath, H. Secretion and metabolism of monomeric human calcitonin: Effects of age, sex, and thyroid damage. Bone Miner. Res. 1986, 1, 339–349, https://doi.org/10.1002/JBMR.5650010407.
- Mazeh, H.; Sippel, R.S.; Chen, H. The role of gender in primary hyperparathyroidism: Same disease, different presentation. Surg. Oncol. 2012, 19, 2958–2962, https://doi.org/10.1245/S10434-012-2378-3.
- Song, E.; Jeon, M.J.; Yoo, H.J.; Bae, S.J.; Kim, T.Y.; Kim, W.B.; Shong, Y.K.; Kim, H.K.; Kim, W.G. Gender-dependent reference range of serum calcitonin levels in healthy Korean adults. Metab. 2021, 36, 365–373, https://doi.org/10.3803/ENM.2020.939.
- Schutte, R.; Nawrot, T.S.; Richart, T.; Thijs, L.; Vanderschueren, D.; Kuznetsova, T.; Van Hecks, E.; Roels, H.A.; Staessen, J.A. Bone resorption and environmental exposure to cadmium in women: A population study. Health Perspect. 2008, 116, 777, https://doi.org/10.1289/EHP.11167.
- Ko, W.C.; Liu, C.L.; Lee, J.J.; Liu, T.P.; Yang, P.S.; Hsu, Y.C.; Cheng, S.P. Negative association between serum parathyroid hormone levels and urinary perchlorate, nitrate, and thiocyanate concentrations in U.S. adults: The national health and nutrition examination survey 2005–2006. PLoS ONE 2014, 9, e115245, https://doi.org/10.1371/JOURNAL.PONE.0115245.
- Soria, M.; Haro, C.G.; Ansón, M.A.; Iñigo, C.; Calvo, M.L.; Escanero, J.F. Variations in serum magnesium and hormonal levels during incremental exercise. Res. 2014, 27, 155–164, https://doi.org/10.1684/MRH.2014.0372.
- Popović, M.; Matana, A.; Torlak, V.; Brdar, D.; Gunjača, I.; Boraska Perica, V.; Barbalić, M.; Kolčić, I.; Punda, A.; Polašek, O.; et al. The effect of multiple nutrients on plasma parathyroid hormone level in healthy individuals. J. Food Sci. Nutr. 2019, 70, 638–644, https://doi.org/10.1080/09637486.2018.1551335.
- Jorde, R.; Saleh, F.; Figenschau, Y.; Kamycheva, E.; Haug, E.; Sundsfjord, J. Serum parathyroid hormone (PTH) levels in smokers and non-smokers. The fifth Tromsø study. J. Endocrinol. 2005, 152, 39–45, https://doi.org/10.1530/EJE.1.01816.
- He, J.L.; Scragg, R.K. Vitamin D, parathyroid hormone, and blood pressure in the National Health and Nutrition Examination Surveys. J. Hypertens. 2011, 24, 911–917, https://doi.org/10.1038/AJH.2011.73.
- Paik, J.M.; Farwell, W.R.; Taylor, E.N. Demographic, dietary, and serum factors and parathyroid hormone in the National Health and Nutrition Examination Survey. Int. 2012, 23, 1727–1736, https://doi.org/10.1007/S00198-011-1776-X.
- Daniels, G.H.; Hegedüs, L.; Marso, S.P.; Nauck, M.A.; Zinman, B.; Bergenstal, R.M.; Mann, J.F.E.; Derving Karsbøl, J.; Moses, A.C.; Buse, J.B.; et al. LEADER 2: Baseline calcitonin in 9340 people with type 2 diabetes enrolled in the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial: Preliminary observations. Diabetes Obes. Metab. 2015, 17, 477–486, https://doi.org/10.1111/DOM.12444.
- Gobba, N.A.E.K.; Hussein Ali, A.; El Sharawy, D.E.; Hussein, M.A. The potential hazardous effect of exposure to iron dust in Egyptian smoking and nonsmoking welders. Environ. Occup. Health 2017, 73, 189–202, https://doi.org/10.1080/19338244.2017.1314930.
- Chen, W.R.; Sha, Y.; Chen, Y.D.; Shi, Y.; Yin, D.W.; Wang, H. Vitamin D, parathyroid hormone, and serum lipid profiles in a middle-aged and elderly Chinese population. Pract. 2014, 20, 556–565, https://doi.org/10.4158/EP13329.OR.
- Yuan, T.J.; Chen, L.P.; Pan, Y.L.; Lu, Y.; Sun, L.H.; Zhao, H.Y.; Wang, W.Q.; Tao, B.; Liu, J.M. An inverted U-shaped relationship between parathyroid hormone and body weight, body mass index, body fat. Endocrine 2021, 72, 844–851, https://doi.org/10.1007/S12020-021-02635-Y.
- Calvo, M.S.; Kumar, R.; Heath, H. Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. Clin. Endocrinol. Metab. 1988, 66, 823–829, https://doi.org/10.1210/JCEM-66-4-823.
- Kemi, V.E.; Kärkkäinen, M.U.M.; Rita, H.J.; Laaksonen, M.M.L.; Outila, T.A.; Lamberg-Allardt, C.J.E. Low calcium:phosphorus ratio in habitual diets affects serum parathyroid hormone concentration and calcium metabolism in healthy women with adequate calcium intake. J. Nutr. 2010, 103, 561–568, https://doi.org/10.1017/S0007114509992121.
- Lederer, E. Regulation of serum phosphate. Physiol. 2014, 592, 3985–3995, https://doi.org/10.1113/JPHYSIOL.2014.273979.
- Grundmann, M.; von Versen-Höynck, F. Vitamin D—Roles in women’s reproductive health? Biol. Endocrinol. 2011, 9, 146, https://doi.org/10.1186/1477-7827-9-146.
- Chapuy, M.C.; Chapuy, P.; Meunier, P.J. Calcium and vitamin D supplements: Effects on calcium metabolism in elderly people. J. Clin. Nutr. 1987, 46, 324–328, https://doi.org/10.1093/AJCN/46.2.324.
- Dawson-Hughes, B.; Dallal, G.E.; Krall, E.A.; Harris, S.; Sokoll, L.J.; Falconer, G. Effect of vitamin D supplementation on wintertime and overall bone loss in healthy postmenopausal women. Intern. Med. 1991, 115, 505–512, https://doi.org/10.7326/0003-4819-115-7-505.
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and calcium to prevent hip fractures in elderly women. Engl. J. Med. 1992, 327, 1637–1642, https://doi.org/10.1056/NEJM199212033272305.
- Ooms, M.E.; Roos, J.C.; Bezemer, P.D.; van der Vijgh, W.J.F.; Bouter, L.M.; Lips, P. Prevention of bone loss by vitamin D supplementation in elderly women: A randomized double-blind trial. Clin. Endocrinol. Metab. 1995, 80, 1052–1058, https://doi.org/10.1210/JCEM.80.4.7714065.
- Kamycheva, E.; Sundsfjord, J.; Jorde, R. Serum parathyroid hormone levels predict coronary heart disease: The Tromsø Study. J. Cardiovasc. Prev. Rehabil. 2004, 11, 69–74, https://doi.org/10.1097/01.HJR.0000114706.27531.01.
- Ilich, J.Z.; Brownbill, R.A.; Tamborini, L.; Crncevic-Orlic, Z. To drink or not to drink: How are alcohol, caffeine and past smoking related to bone mineral density in elderly women? Am. Coll. Nutr. 2002, 21, 536–544, https://doi.org/10.1080/07315724.2002.10719252.
- Vantyghem, M.C.; Danel, T.; Marcelli-Tourvieille, S.; Moriau, J.; Leclerc, L.; Cardot-Bauters, C.; Docao, C.; Carnaille, B.; Wemeau, J.L.; D’Herbomez, M. Calcitonin levels do not decrease with weaning in chronic alcoholism. Thyroid 2007, 17, 213–217, https://doi.org/10.1089/THY.2006.0216.
- Schuster, R.; Koopmann, A.; Grosshans, M.; Reinhard, I.; Spanagel, R.; Kiefer, F. Association of plasma calcium concentrations with alcohol craving: New data on potential pathways. Neuropsychopharmacol. 2017, 27, 42–47, https://doi.org/10.1016/J.EURONEURO.2016.11.007.
- Ilias, I.; Paparrigopoulos, T.; Tzavellas, E.; Karaiskos, D.; Meristoudis, G.; Liappas, A.; Liappas, I. Inpatient alcohol detoxification and plasma calcitonin (with original findings). J. Nucl. Med. 2011, 14, 177–178.
- Ljunghall, S.; Joborn, H.; Roxin, L.E.; Skarfors, E.T.; Wide, L.E.; Lithell, H.O. Increase in serum parathyroid hormone levels after prolonged physical exercise. Sci. Sport. Exerc. 1988, 20, 122–125, https://doi.org/10.1249/00005768-198820020-00004.
- Diaz-Castro, J.; Mira-Rufino, P.J.; Moreno-Fernandez, J.; Chirosa, I.; Chirosa, J.L.; Guisado, R.; Ochoa, J.J. Ubiquinol supplementation modulates energy metabolism and bone turnover during high intensity exercise. Food Funct. 2020, 11, 7523–7531, https://doi.org/10.1039/D0FO01147A.
- Lin, L.L.; Hsieh, S.S. Effects of strength and endurance exercise on calcium-regulating hormones between different levels of physical activity. Mech. Med. Biol. 2005, 5, 267–275, https://doi.org/10.1142/S0219519405001461.
- Åkesson, A.; Bjellerup, P.; Lundh, T.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Skerfving, S.; Vahter, M. Cadmium-induced effects on bone in a population-based study of women. Health Perspect. 2006, 114, 830–834, https://doi.org/10.1289/EHP.8763.
- Ahmed, S.; Rekha, R.S.; Ahsan, K. Bin; Doi, M.; Grandér, M.; Roy, A.K.; Ekström, E.C.; Wagatsuma, Y.; Vahter, M.; Raqib, R. Arsenic exposure affects plasma insulin-like growth factor 1 (IGF-1) in children in rural Bangladesh. PLoS ONE 2013, 8, e81530, https://doi.org/10.1371/JOURNAL.PONE.0081530.
- Mazumdar, I.; Goswami, K.; Ali, M.S. Status of serum calcium, vitamin D and parathyroid hormone and hematological indices among lead exposed jewelry workers in Dhaka, Bangladesh. Indian J. Clin. Biochem. 2017, 32, 110, https://doi.org/10.1007/S12291-016-0582-9.
- Potula, V.; Henderson, A.; Kaye, W. Calcitropic hormones, bone turnover, and lead exposure among female smelter workers. Environ. Occup. Health 2005, 60, 195–204, https://doi.org/10.3200/AEOH.60.4.195-204.
- Rignell-Hydbom, A.; Skerfving, S.; Lundh, T.; Lindh, C.H.; Elmståhl, S.; Bjellerup, P.; Jünsson, B.A.; Strümberg, U.; Akesson, A. Exposure to cadmium and persistent organochlorine pollutants and its association with bone mineral density and markers of bone metabolism on postmenopausal women. Res. 2009, 109, 991–996, https://doi.org/10.1016/J.ENVRES.2009.08.008.
- Guo, Y.L.; Lin, C.J.; Yao, W.J.; Ryan, J.J.; Hsu, C.C. Musculoskeletal changes in children prenatally exposed to polychlorinated biphenyls and related compounds (Yu-Cheng children). Toxicol. Environ. Health 1994, 41, 83–93, https://doi.org/10.1080/15287399409531828.
- Di Nisio, A.; Rocca, M.S.; De Toni, L.; Sabovic, I.; Guidolin, D.; Dall’Acqua, S.; Acquasaliente, L.; De Filippis, V.; Plebani, M.; Foresta, C. Endocrine disruption of vitamin D activity by perfluoro-octanoic acid (PFOA). Rep. 2020, 10, 1–12, https://doi.org/10.1038/s41598-020-74026-8.
- Koroglu, B.K.; Ersoy, I.H.; Koroglu, M.; Balkarli, A.; Ersoy, S.; Varol, S.; Tamer, M.N. Serum parathyroid hormone levels in chronic endemic fluorosis. Trace Elem. Res. 2011, 143, 79–86, https://doi.org/10.1007/S12011-010-8847-2.
- Skórka-Majewicz, M.; Goschorska, M.; Żwierełło, W.; Baranowska-Bosiacka, I.; Styburski, D.; Kapczuk, P.; Gutowska, I. Effect of fluoride on endocrine tissues and their secretory functions—Review. Chemosphere 2020, 260, 127565, https://doi.org/10.1016/j.chemosphere.2020.127565.
