Neuroendocrine neoplasms (NEN) consist of a very heterogenous group of tumors, contributing to large differences in patients’ disease burden, symptomatology, clinical and objective responses to different treatments, and prognosis. Liver-directed treatments for NELM can be divided into two categories: ablative localized treatments, e.g., radiofrequency ablation (RFA) or microwave ablation (MWA); or trans-arterial treatments, e.g., trans-arterial (bland) embolization (TAE), trans-arterial chemoembolization (TACE) and trans-arterial radioembolization (TARE). The latter technique is also known as selective internal radiation therapy (SIRT). Radioembolization is a more commonly used and simplified term but also a misnomer. Contrary to TAE and TACE, the primary effect is not to embolize vasculature and induce ischemia but to deliver high doses of radiation to tumor tissue via trans-arterial implantation.
- NEN
- radioembolization
- SIRT
- neuroendocrine tumor
1. Introduction
In accordance with the most recent WHO/ENETS criteria, tumor grading is the most common denominator for survival: grade 1 and 2 neuroendocrine tumors (G1-/G2NET) are regarded as well- to moderately differentiated tumors with a low Ki67 index (<3% and 3–20%, respectively); grade 3 NET (G3NET) as well- or moderately differentiated NET with high Ki67 index >20%; and neuroendocrine carcinomas (NEC) as poorly differentiated and with highly proliferative tumors (Ki67 index is most commonly >55%) [1][2][3]. However, within its heterogeneity, a well-established negative factor for survival for NEN patients is the presence of neuroendocrine liver metastases (NELM) [4]. Unfortunately, at diagnosis, 21% of all G1NET, 30% of all G2NET and 50% of all G3NET already have distant metastases, of which the liver is the most commonly affected [5][6]. In the presence of NELM, Frilling et al. provided an easy method to categorize liver involvement into three groups, based on tumor distribution in the liver [7]: from a ‘simple pattern’ (NELM involves 1–2 liver segments) to a ‘complex pattern’ (extensive unilobar disease with limited disease in the contralateral lobe) to a ‘diffuse pattern’ (bilobar or miliary disease). Whereas these ‘simple’ and ‘complex’ patterns allow surgical resection, the ‘diffuse’ pattern does not. Unfortunately, 60–70% of patients with NELM reside in the ‘diffuse pattern’ group, illustrating the clinical need for liver-directed treatments in light of the limited systemic options for NENs.
Within trans-arterial treatments for NELM, radioembolization has gained a lot of attention over the last decade and reports high tumor objective response rates and limited toxicities [8]. As illustrated by the European Neuroendocrine Tumor Society (ENETS) guideline from 2016 and the European Society for Medical Oncology (ESMO) guideline from 2020, the role of radioembolization has extended, including early application as a tumor debulking treatment or as a salvage treatment in selected cases, after the failure of systemic treatments [4][9]. As NEN and NELM development are highly variable between individuals, the application of radioembolization needs to be determined on a case-by-case basis through discussions by multidisciplinary tumor boards (MDT).
2. How Is Radioembolization Performed?
| Clinical Assessment | Laboratory Testing | Imaging Work-Up |
|---|---|---|
| Minimal | ||
| ECOG performance score | Bilirubin, ALP, AST, ALT, albumin | gdMRI/CECT for intrahepatic tumor load 1 |
| Signs of hepatic dysfunction (Child–Pugh score) | Creatinine, eGFR | Early-phase CECT for arterial vasculature |
| NET hormone-related symptoms | Tumor markers (e.g., CgA, gastrin) | |
| Additional | ||
| In selected cases, Fibroscan or gastroscopy to assess esophageal varices | Hb, hematocrit, WBC, platelets | SSTR-PET/CT for total body tumor load 1 |
| Coagulation (e.g., Prothrombin time or INR) | FDG-PET/CT for tumor grade distinction, excluding aggressive disease. |
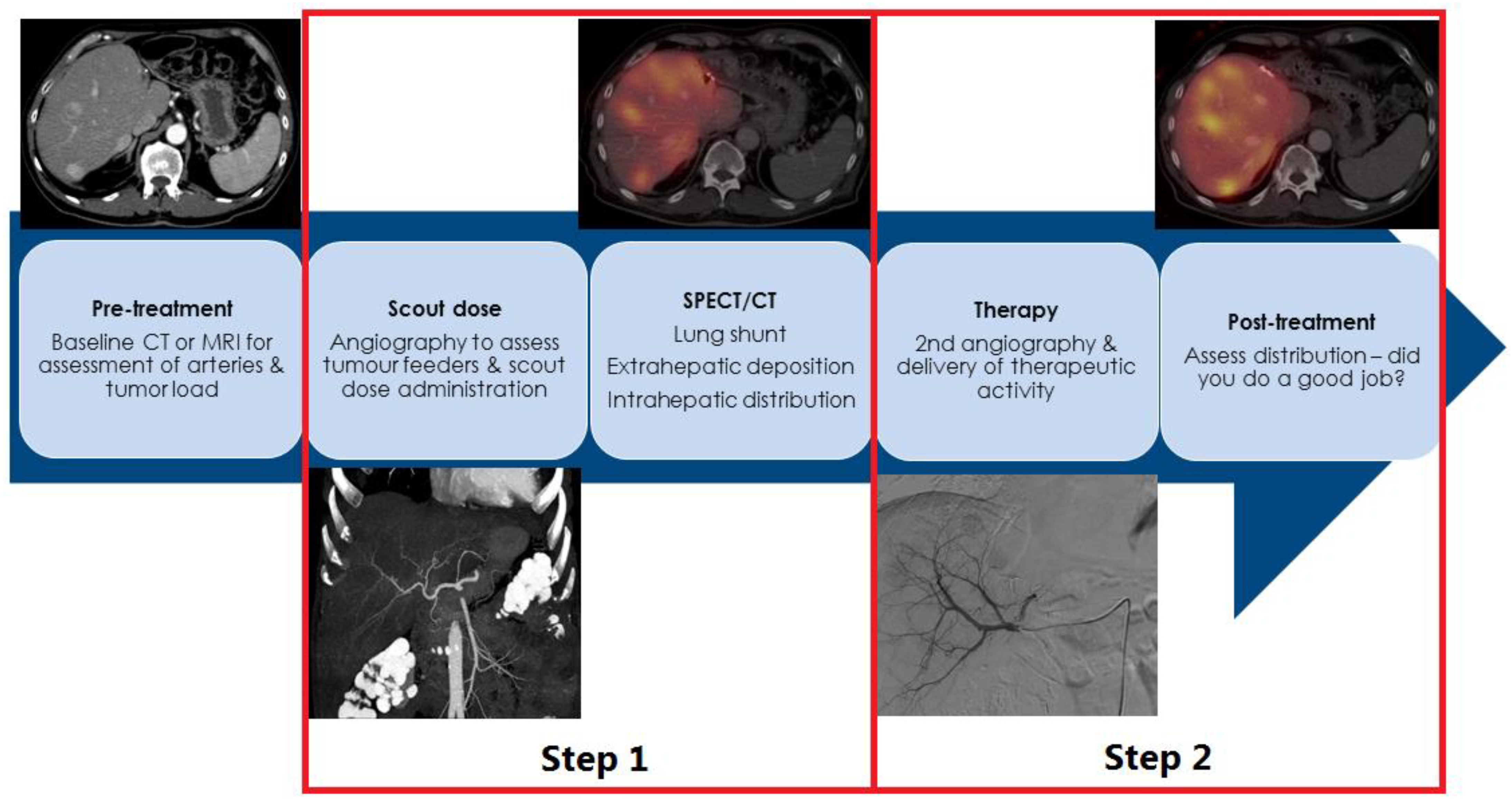
3. SIRT in NEN: Salvage Setting
Table 2 summarizes the most important scientific evidence available to date for salvage radioembolization.
| Year | N | ORR * | DCR * | PFS | OS | REILD | |
|---|---|---|---|---|---|---|---|
| % | % | Months | Months | n (%) | |||
| Devcic et al. † [8] | 2014 | 435 | 50 | 86 | NR | 28.5 | NR |
| Peker et al. [13] | 2015 | 38 | 46 | 83 | NR | 39 | 0 |
| Barbier et al. [14] | 2016 | 54 | 54 | 94 | NR | 34.8 | 1 (1.8) |
| Braat et al. [10] | 2019 | 244 | 16 | 91 | NR | 31 | 2 (0.8) |
| 43 | 91 | ||||||
| Schaarschmidt et al. [15] | 2022 | 297 | 41.3 | 83.5 | 15.9 | 30.6 | 2 (0.8) |
| Wong et al. [16] | 2022 | 170 | 36 | 69 | 25 | 33 | 1 (0.6) |
4. Radioembolization in Earlier Lines or Combinations Treatments
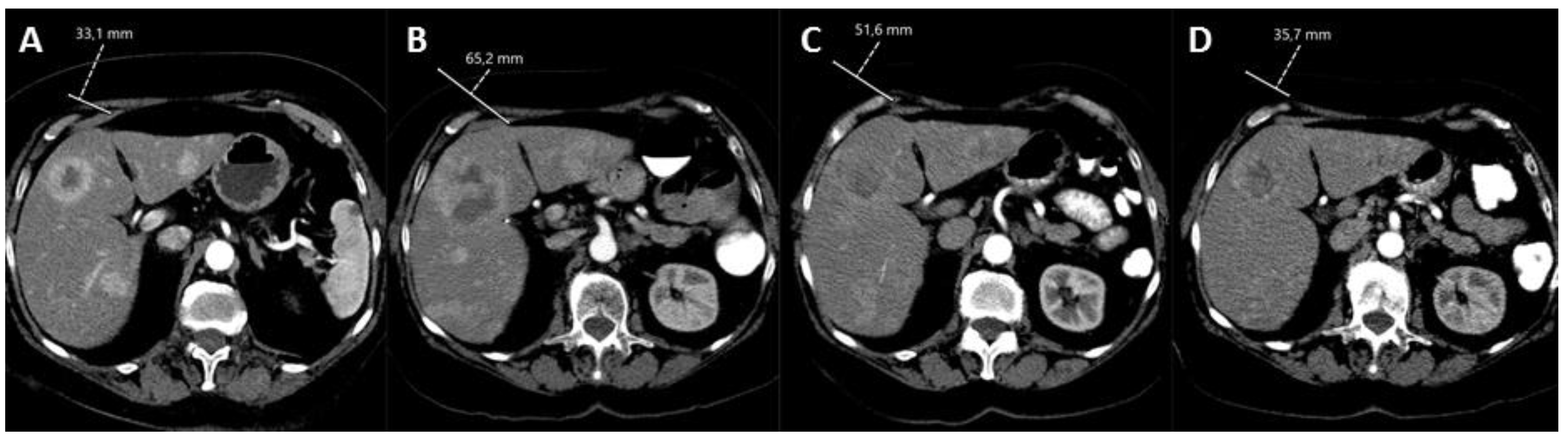
5. Conclusions
Hepatic radioembolization is safe and effective as a monotreatment in NEN. Based on current evidence, the exact application of radioembolization in NEN care remains unknown, and the scientific debate on suggested long-term toxicities remains unresolved. The application of radioembolization should be considered on a case-by-case basis through multidisciplinary discussion. Upcoming clinical and technical developments in the field will ensure a more promising role for radioembolization in NEN care.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14143415
References
- Bosman, F.T.; Carneiro, F. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System, 4th ed.; IARC: Lyon, France, 2010.
- Capelli, P.; Fassan, M.; Scarpa, A. Pathology-grading and staging of gep-nets. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 705–717.
- Heetfeld, M.; Chougnet, C.N.; Olsen, I.H.; Rinke, A.; Borbath, I.; Crespo, G.; Barriuso, J.; Pavel, M.; O’Toole, D.; Walter, T.; et al. Characteristics and treatment of patients with g3 gastroenteropancreatic neuroendocrine neoplasms. Endocr. Relat. Cancer 2015, 22, 657–664.
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. Enets consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (nen) and nen of unknown primary site. Neuroendocrinology 2016, 103, 172–185.
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after carcinoid: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the united states. J. Clin. Oncol. 2008, 26, 3063–3072.
- Pavel, M.; Baudin, E.; Couvelard, A.; Krenning, E.; Oberg, K.; Steinmuller, T.; Anlauf, M.; Wiedenmann, B.; Salazar, R.; Barcelona Consensus Conference participants. Enets consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012, 95, 157–176.
- Frilling, A.; Modlin, I.M.; Kidd, M.; Russell, C.; Breitenstein, S.; Salem, R.; Kwekkeboom, D.; Lau, W.Y.; Klersy, C.; Vilgrain, V.; et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014, 15, e8–e21.
- Devcic, Z.; Rosenberg, J.; Braat, A.J.A.T.; Techasith, T.; Banerjee, A.; Sze, D.Y.; Lam, M.G.E.H. The efficacy of hepatic 90y resin radioembolization for metastatic neuroendocrine tumors: A meta-analysis. J. Nucl. Med. 2014, 55, 1404–1410.
- Pavel, M.; Oberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860.
- Braat, A.J.A.T.; Kappadath, S.C.; Ahmadzadehfar, H.; Stothers, C.L.; Frilling, A.; Deroose, C.M.; Flamen, P.; Brown, D.B.; Sze, D.Y.; Mahvash, A.; et al. Radioembolization with (90)y resin microspheres of neuroendocrine liver metastases: International multicenter study on efficacy and toxicity. Cardiovasc. Intervent. Radiol. 2019, 42, 413–425.
- Cholapranee, A.; van Houten, D.; Deitrick, G.; Dagli, M.; Sudheendra, D.; Mondschein, J.I.; Soulen, M.C. Risk of liver abscess formation in patients with prior biliary intervention following yttrium-90 radioembolization. Cardiovasc. Intervent. Radiol. 2015, 38, 397–400.
- Reinders, M.T.M.; Mees, E.; Powerski, M.J.; Bruijnen, R.C.G.; van den Bosch, M.A.A.J.; Lam, M.G.E.H.; Smits, M.L.J. Radioembolisation in Europe: A Survey Amongst CIRSE Members. Cardiovasc. Intervent. Radiol. 2018, 41, 1579–1589.
- Peker, A.; Cicek, O.; Soydal, C.; Kucuk, N.O.; Bilgic, S. Radioembolization with yttrium-90 resin microspheres for neuroendocrine tumor liver metastases. Diagn. Interv. Radiol. 2015, 21, 54–59.
- Barbier, C.E.; Garske-Roman, U.; Sandstrom, M.; Nyman, R.; Granberg, D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1425–1431.
- Schaarschmidt, B.M.; Wildgruber, M.; Kloeckner, R.; Nie, J.; Steinle, V.; Braat, A.J.A.T.; Lohoefer, F.; Kim, H.S.; Lahner, H.; Weber, M.; et al. 90y radioembolization in the treatment of neuroendocrine neoplasms: Results of an international multicenter retrospective study. J. Nucl. Med. 2022, 63, 679–685.
- Wong, T.Y.; Zhang, K.S.; Gandhi, R.T.; Collins, Z.S.; O’Hara, R.; Wang, E.A.; Vaheesan, K.; Matsuoka, L.; Sze, D.Y.; Kennedy, A.S.; et al. Long-term outcomes following 90y radioembolization of neuroendocrine liver metastases: Evaluation of the radiation-emitting sir-spheres in non-resectable liver tumor (resin) registry. BMC Cancer 2022, 22, 224.
- Braat, A.J.A.T.; Kwekkeboom, D.J.; Kam, B.L.R.; Teunissen, J.J.M.; de Herder, W.W.; Dreijerink, K.M.A.; van Rooij, R.; Krijger, G.C.; de Jong, H.W.A.M.; van den Bosch, M.A.A.J.; et al. Additional hepatic 166ho-radioembolization in patients with neuroendocrine tumours treated with 177lu-dotatate; a single center, interventional, non-randomized, non-comparative, open label, phase ii study (hepar plus trial). BMC Gastroenterol. 2018, 18, 84.
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with (177)lu-dotatate: An analysis of the netter-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382.
- Braat, A.J.A.T.; Bruijnen, R.C.G.; van Rooij, R.; Braat, M.N.G.J.A.; Wessels, F.J.; van Leeuwaarde, R.S.; van Treijen, M.J.C.; de Herder, W.W.; Hofland, J.; Tesselaar, M.E.T.; et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (hepar plus): A single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. 2020, 21, 561–570.
