2. Electrospinning: Applications
2.1. Medical: Tissue Engineering
To repair the extracellular matrix that has been damaged, a wide range of scaffold-based tissue engineering technologies and materials have been developed. During the last decade, researchers have concentrated on creating biodegradable polymeric membranes that can be used to regenerate periodontal tissue [
38]. Due to their diversity in material selection and control over scaffold features, electrospun scaffolds have been employed in tendon/ligament, neural, vascular, cartilage, and bone applications. Tissue engineering (TE) is a fascinating field that combines engineering and biology to generate or repair organ tissue. It uses cells, biomaterials, and biomolecules as its primary tools. By using TE, organ transplantation may be avoided. The seeding of cells into the material structure of the artificial materials given by TE to assist 3D tissue creation is frequently enhanced. Scaffolding methods abound, but electrospinning (ES) stands out for its ability to construct nonwoven fibrous structures with dimensional components equivalent to ECM fibers. Several studies have shown that the ES approach may be a feasible scaffolding method for tissue-engineering applications [
39].
A tissue substitute’s architecture has a significant role in regulating tissue development [
40]. For the ideal scaffold to function, it must meet a range of frequently competing requirements, including adequate surface area, optimal levels, and sizes of porosity to enable cell migration, and degradation rates that closely mimic the regeneration rates of the desired natural tissue [
41]. Even though there are many different forms of synthetic scaffolding, a few stand out as particularly promising. According to the extensive usage of the ES process, structures made of ES fibers come under this category [
42]. Scaffolds created using ES imitate the natural extracellular matrix (ECM) using nano- and microfibers, making them a simple and versatile technique. Despite their many benefits and widespread usage, the limited cell infiltration and low mechanical strength of electrospun scaffolds make them unsuitable for all but the most demanding applications. A variety of research groups have addressed the restrictions as mentioned above [
41].
Controlled drug release using electrospun matrices, including antibiotics, DNA, and proteins, are possible for applications in tissue engineering. To regenerate cartilage, tissue engineering has employed collagen sponges, hydrogel scaffolds, and gelatin-based microspheres. A similar structure to the ECM in native cartilage has been explored in nanofibers made from synthetic, natural, and new combinations of polymers [
43]. Electrospun nanofibers may increase scaffold properties including cell-matrix interaction and chondrogenic differentiation. The large-surface-area electrospun PCL scaffolds facilitated cell-matrix interaction and chondrocyte formation without growth factors [
44].
Clinically, tendon and ligament injuries, common in young athletes, provide a considerable difficulty because of their inherent inability to recover. As a result of the healing process, scar-like tissue often develops with inferior mechanical qualities. A new method for tendon and ligament regeneration is possible via tissue engineering. From the nanofibrous structure developed through the electrospinning process used for scaffolds, researchers have been able to mimic the collagen fiber bundles of natural tissues in the regeneration of ligaments and tendons [
45]. The mechanical characteristics of normal healthy tendons are extremely anisotropic because they are formed of parallel arrays of tightly packed collagen fibers. Therefore, nanofiber-based scaffolds that mirror the anisotropic structure of tendon and ligament tissue are potential options for tissue engineering. The cellular behavior of stem cells and committed fibroblasts has been studied with the nanofiber’s diameter and orientation [
46].
A three-dimensional multilayered scaffold developed using polyethylene-glycol-based porous chitosan and nanofibrous mats [
4]. Compared to the 3D random and porous control scaffolds, the 3D-aligned nanofiber-embedded scaffold exhibited a cell filtration depth of 45 m. Consequently, cell viability on the 3D-AL scaffold was much greater than on the 3D-RD scaffold 7 days after seeding (OD value of around 2.2 and about 1.5, respectively). As opposed to random nanofiber scaffolds, tenomodulin expression was maximum when platelet-derived growth factor-BB was immobilized in a gradient, aligned nanofiber scaffold (18.501.45). In an in vivo experiment, the supraspinatus tendon reinforced with an electrospun PLGA nanofibrous scaffold exhibited a higher Young’s modulus (48.6 MPa) than the supraspinatus tendon that had just seen primary healing (3.79 MPa) [
47]. We also demonstrated an electrospinning approach that used PCL scaffolds seeded with human ADSCs to improve cell penetration and collagen deposition. Compared to nonaligned multilayered scaffolds, the fold change in collagen type III and tenomodulin increased from 2 to 2.5 and 3 to 25, respectively, indicating improved tendon-related gene expression.
For a variety of cardiovascular illnesses, there is increasing need for tissue-engineered vascular grafts (TEVGs) to repair or bypass damaged arteries. To create TEVGs from tissue skeletons, biopolymers and biodegradable synthetic polymers have been used. The inability to replicate natural tissue mechanical properties and the necessity for long-term patency and development needed for in vivo function remain unclear. Scaffolds are often made by electrospinning, which has the potential to alleviate these issues. TEVGs from humans have not yet been tested using this technique. The first human trials of tissue-created blood vessels for high-pressure arteries have been completed.
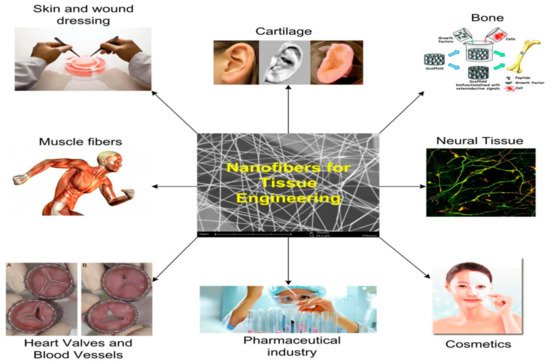
Tissue-engineered scaffolds are injected into the urinary tract organs for reconstructive purposes through electrospun scaffolds in patients with a sick bladder who have lost their ability to operate as a compliant and low-pressure reservoir must undergo augmentation cystoplasty. It is possible to regenerate some lost bladder tissue using electrospun scaffolds as shown in Figure 4. An electrospun scaffold may be used to replace an affected urethral wall with a stricture lesion, either as a direct or indirect replacement.
Figure 4. A schematic of the applications of tissue engineering [
48].
2.2. Renewable Energy
Electrospinning is a simple and low-cost method for producing nanofibers. Inorganic materials, mainly metal oxides, may be created and electrospun to improve their conducting and ceramic characteristics, a massive boon for energy devices. Sustainable energy and habitats will be made possible in the future when it comes to advances in nanotechnology. Nanofiber-structured materials can be extremely effective in addressing energy, health, and environmental concerns. Innovative methods for harvesting renewable energy using cutting-edge technology are made possible by advances in nanotechnology. Semiconductors made from electrospinning nanofibers, in particular, are being touted as a potential solution to our energy woes. Electrospinning, a simple and inexpensive method for creating nanofibers, is an effective way to do so. It is also possible to create nanofibers from a wide range of materials, including organics and inorganics, using electrospinning [
49].
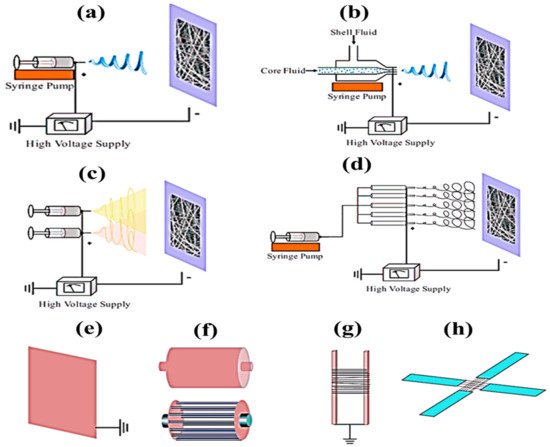
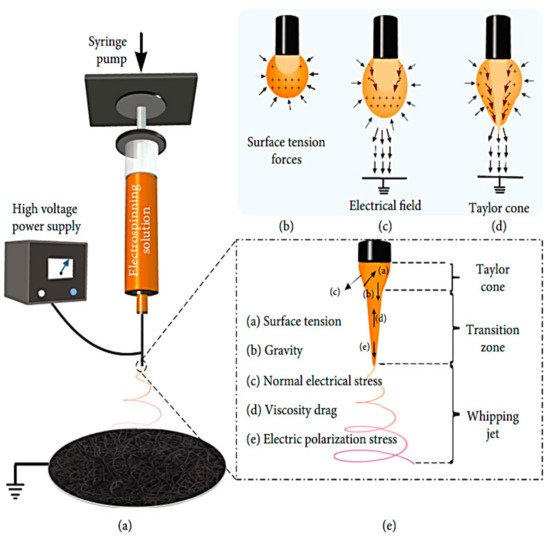
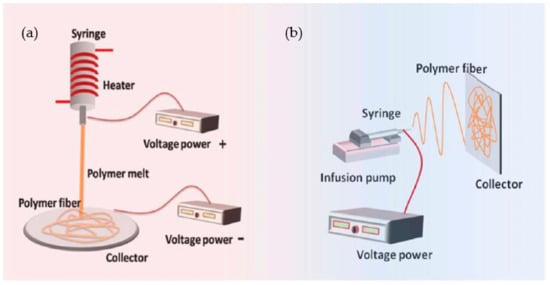
In electrospinning, a polymer solution is charged and ejected under a high-voltage electric field in order to create micro- and ultrafine fibers (in nanometers). Polymer solution is spun into yarn using an electrospinning process governed by electrohydrodynamic principles. Electrospinning’s basic setup may be seen in the image. Here, the polymer solution is held in a reservoir (usually in the form of a syringe) and is applied to an electrode through the pump, high-voltage power source, and collection device described above [
50]. Electrostatic repulsion causes the droplet to be stretched when high voltage is given to a polymer liquid droplet. A jet of liquid gushes out from under the collector at this moment. Taylor cone is the name given to the eruptive site. Taylor cone geometry is a compromise between the Maxwell stresses that are transmitted into melting fluid and the surface tension that maintains meniscal shape. In this case, the jet does not break up into droplets but creates an electrically charged laminar jet, which elongates owing to electrostatic repulsion. This is the mechanism of electro-spraying. In solution electrospinning, or in melt electrospinning, the jet dries or cools sufficiently to become solid, and a nanoscale fiber is generated (4).
Fibers with distinct structures and characteristics can be made by altering the spinneret or the solution. Using electrospinning in a number of ways is a viable option. Two solutions can be injected into the spinneret tip for co-axial electrospinning. The electrospinning jet’s Taylor cone’s sheath fluid is hypothesized to act as a carrier for the inner fluid, bringing it into contact with the outer fluid [
51]. To make core-shell or composite fibers without changing the spinneret is possible in emulsions. However, because of the increased number of factors that must be taken into account while generating the emulsion, these fibers are often more difficult to make than coaxial spinning [
52]. Polymer melt electrospinning, on the other hand, does not require volatile solvents because it uses polymer melts. Semicrystalline fibers are made from a variety of polymers, including PE, PET, and PP. Electrospinning with a syringe or spinneret, a high-voltage source, and a collector is quite similar to conventional electrospinning. Resistance heating, flowing fluids, air heating, or layering are the most common methods for melting polymers [
53].
Electrospinning is a valuable technology for making micro- and nanoscale fibers in both laboratories and industry. Numerous polymers, including single and mixed polymers, are employed in electro-fiber production. Electrospinning’s intricate hierarchical structure under regulated calcination is one of its unique advantages [
54]. There are several ways to use the unique features for energy, environment, and tissue engineering. There are a number of energy-related applications for electrospinning technology, including the production of batteries, dye-sensitive solar cells, and supercapacitors [
55].
2.3. Face Mask
SARS-CoV-2 may be prevented from spreading from person to person by wearing a face mask made of ultrafine fibers with a diameter of a few tens of nanometers or less. For example, the electrospun ultrafine fiber filter can be antiviral, transparent, and degradable in addition to virus-blocking. This is an essential component in the battle against epidemics. Filtration performance and reusability of electrospun ultrafine fiber-based masks: a production technique [
56,
57,
58].
Since the dawn of humans, we have relied on textiles for everything from clothing to shelter. On the other hand, fibers are the fundamental building blocks of these substances. According to this viewpoint, nanofibers, as active layers in face masks, may protect patients against the new coronavirus sickness (COVID-19). A few things should be known about these electrospun nanofiber face masks in particular, including how they work and how you may build your own at home, all in one short, but essential, letter. Fine tiny sieves, which operate as active layers in face masks, are what give them their functionality. A microscopic sieve is composed of entangled mats comprised of extremely fine fibers capable of providing complicated routes for airborne particles, viruses, and bacteria. Three distinct particles may be prevented from reaching the user via four different techniques while the face mask is in use. Micro-, macro-, and nanoparticles are all categorized independently in Figure 5.
Figure 5. Various types of protective masks [
56].
The three particle categories now include charged particles. Larger macroparticles (over 600 nm) cannot pass through the filters and are intercepted outside the masks. Microfine particles (300–600 nm) may pass through the mat pores of the mask sieve, but they are more likely to smash against the fiber walls (like any item traveling in a non-straight route at high speeds). The particle’s mass and velocity govern this, preventing it from reaching the wearer. This is called the impact/collision mechanism. Due to their tiny size, nanoparticles (below 300 nm) may readily pass through pores without interacting with the pore walls but are quickly assaulted by the air molecules surrounding them. Diffusion-based capture is used to capture such particles, which only occurs in finer fibers (nanofibers) and branching nanofibers.
Electrospun nanofibers are more efficient here. However, small particles (300 nm) that do not obey the impact/collision process, or the diffusion-based capture method are difficult to filter in many face masks. To delay such particles and allow them to follow one of the processes, numerous layers of mats are required. Multiple filtration layers present a new technical challenge for the final product’s breathability. Engineers must handle the opposing needs of air filterability and air breathability to achieve the perfect performance of a face mask. With proper electrospinning process management, electrospun nanofibers offer the necessary equilibrium. By using electrostatic filtration, the last process, particles attracted by their negative charges are prevented from reaching the wearer by filtration mats that are charged [
57].
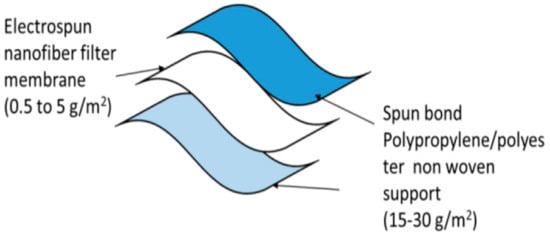
Two polyester/polypropylene nonwovens plus a fine-fiber filtration membrane make up a face mask’s three or more layers of composite structures shown in Figure 6. As an additional layer of activated carbon cloth, VOCs may be removed (Singh et al. 2002). Additionally, the wearer’s nose and mouth are protected by a layer of modacrylic to keep the mask in place and prevent it from falling apart. As a result, most commercial masks are constructed using four to five layers of cloth.
Figure 6. Three-layered nanofiber masks [
57].
The skin contact layer should be made of soft nonwoven fabric that is hydrophilic and retains cough droplets. The outermost membrane may be a blood/liquid repellent. The middle layer membrane is a high-efficiency performance filter membrane with tiny pores [
58].
SARS-CoV-2 airborne transmission is essential in COVID-19 dissemination. We created electrospun nanofibrous air filters for personal protection equipment and the interior environment to safeguard public health. The electrospun air filters featured substantially smaller pores than surgical and textile masks due to ultrafine nanofibers (300 nm) (a couple of microns versus tens to hundreds of microns). In prior investigations, coronavirus strains were utilized to create aerosols for filtering efficiency testing as shown in
Figure 7. The electrospun air filters surpassed several conventional face masks by catching up to 99.9% of coronavirus aerosols. A comparison of the filtration effectiveness of coronavirus aerosols and NaCl aerosols was also performed. It was shown that NaCl aerosols might be employed as an effective surrogate for coronavirus aerosols in filtering experiments using air filters and face masks of various sizes and efficiency. To stop SARS-CoV-2 transmission, we developed electrospun nanofibrous air filters. It is also possible to learn how air filters catch coronavirus aerosols by studying the removal effectiveness of NaCl [
59].
Figure 7. Coronavirus aerosols [
59].
2.4. Melt Electrospinning
Melt electrowriting is a new form of an additive manufacturing (AM) process which is used to fabricate new thermoplastic microfiber scaffolds used in tissue engineering [
60]. It is presently a newly emerging technique used for fabricating micro-/nanofibers. It could precisely control the deposition of newly synthesized polymer microfibers through electrohydrodynamic jet deposition on the surface collector via a computerized controlled collector [
61]. Melt electrospinning produces different fibrous shapes and structures from new combinations of polymeric solutions for different applications such as textiles, filtration, and tissue engineering. The PCL melt electrospinning (ME) is shown in the fibers in
Figure 8a produces a smooth and high crystallinity, whereas the PCL solution electrospinning (SE) is shown in
Figure 8b produce fibers exhibiting a high porous and more amorphous nature than the PCL ME fibers. The effectiveness of different process parameters of both the ME and SE techniques used for developing morphology and fiber diameters were researched [
62].
Figure 8. (
a) Melt and (
b) Solution Electrospinning [
62].
The PCL melt fibers were a more suitable solution in fibers (Lian and Meng 2017). The morphological analysis revealed high porosity of solution/melt electrospun mats (94.78%). Higher filtration efficiencies were reached when both electrospinning techniques were applied simultaneously [
64]. The melt electrospinning writing (MEW) was used to fabricate scaffolds with good mechanical strength and controllable structure and for regeneration of bone. The gelatin nanofibers were incorporated in scaffolds to become hydrophilic and enhance the mechanical strength [
59].
MES is a new method for developing amorphous and water-soluble drugs. If the water contents were better controlled, then it would enable minimizing the MES operating temperature, significantly enhance the plasticization and develop extra pressure in a cylindrical syringe, which minimizes thermal depletion of the incorporated drug [
65]. The ME eliminated the use of aggressive solvents and sulfonation which is toxic to PEEKs. This process could replace the current solution fabrication techniques used in the various fields for PEEK fiber membranes [
66].
2.5. Melt Electrowriting
A random copolymer setup with controlled molecular weights and different compositions enables fabricating the well-defined structures [
67]. Melt electrowriting (MEW) is an additive fiber-manufacturing process that is capable of fabricating thin-microfiber thermoplastic-based scaffolds for tissue engineering. It produces micron-scale biocompatible constructs through electrodynamic jet deposition with a high level of orientation control over fiber deposition on the collector. The rotating cylindrical collector to produce the tubular geometry could fabricate the anatomical tissues such as blood vessels [
68].
2.6. Wound Dressing
Electrospun wound dressings are among the most prominent. Wound-healing medications can be incorporated into these dressings in a variety of ways, depending on the patient’s specific needs [
69]. Electrospinning can be used to create a wound dressing that mimics the skin’s natural extracellular matrix (ECM) in order to enable cellular communication, adhesion, migration, and proliferation, as well as cellular adhesion, migration, and proliferation. It is possible to produce submicron fibers with a high surface-to-volume ratio that resembles the structural features of ECM using electrospinning as an alternative method [
70].
Electrospun nanofiber mats are capable of filtering even the tiniest particles or molecules, thanks to their extremely narrow pores. Because of this, they can be used in conjunction with other materials, such as those with antibacterial properties or high surface-to-volume ratios, to further treat the filtered material. Fine nanofiber mats are susceptible to mechanical damage; however, reinforcement options include inserting them in composites or attaching them to more mechanically stable layers. Stabilizing electrospun nanofiber mats by 3D printing hard polymer layers on top of them is generally feasible. In order to avoid delamination of the nanofiber mat and damage to it by the hot nozzle, here we report on the reversed process (i.e., first 3D printing a rigid scaffold and then electrospinning the nanofiber mat on top of it), which is more difficult to perform [
71]. The antibacterial activities increased with increasing ZnO nanoflower contents, and these 3D-printing filaments were better against Gram-positive than Gram-negative bacteria because of differences in their cell walls [
72,
73].