Hydrogels are water-enriched polymeric biomaterials used as scaffolds that mimic the extracellular matrix and are employed in various tissue engineering applications. Interestingly, the hydrogels can be tuned by altering the functional groups of the parent polymeric backbone, resulting in structural rearrangements depending on the physiochemical alterations in the surrounding medium and forming intelligent/smart/stimuli-responsive hydrogels. Myocardial infarction (MI) causes impaired cardiac function due to the loss of cardiomyocytes following an ischemic attack. Intelligent hydrogels offer promising solutions for post-MI cardiac tissue therapy to aid in structural support, contractility, and targeted drug therapy. Hydrogels are porous hydrophilic matrices used for biological scaffolding, and upon the careful alteration of ideal functional groups, the hydrogels respond to the chemistry of the surrounding microenvironment, resulting in intelligent hydrogels.
- intelligent hydrogels
- tissue engineering
- cardiac regeneration
1. Intelligent Hydrogels and Cardiac Tissue Engineering
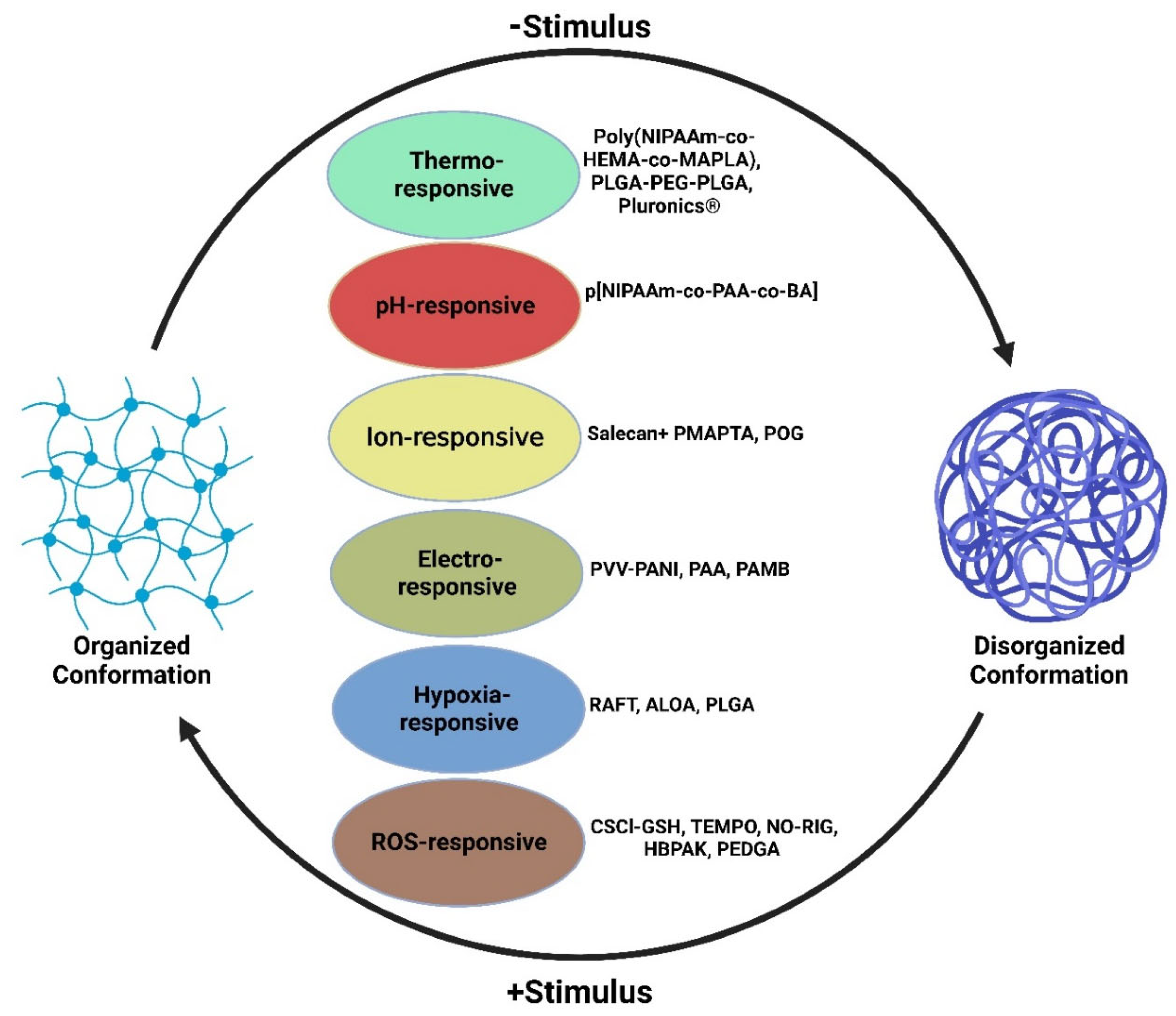
2. Temperature-Responsive Hydrogels
3. pH-Responsive Hydrogel
4. Ion-Responsive Hydrogels
| Type of Smart Hydrogel | Molecular Compound | Function of Hydrogel | Advantages | Limitations | References |
|---|---|---|---|---|---|
| Temperature -Responsive | Poly(NIPAAm-co-HEMA-co-MAPLA) | Provides mechanical support to left ventricular wall via thickening and decreasing mechanical stress | Biodegradable through modification of copolymers, effective site-specific drug delivery, decrease in systemic side effects, evade toxic solvents, high solvent swelling | Decreased pH via acidic degradation, lacks biocompatibility | [38][43][44] |
| Temperature-Responsive | PLGA-PEG-PLGA | Liquid between the temperatures of 2 °C and 15 °C and transitions into a gel at body temperature | Biocompatible, water-soluble, and non-immunogenic, gradual drug release for both hydrophobic and hydrophilic drugs | Hydrophobic/hydrophilic imbalance could lead to no phase change, narrow gel transition temperature window | [17][18][19][20][21][22][23][24][25][26][27][32][33][34][35][36][37][38][39][40] |
| Temperature -Responsive | Pluronics® | At concentration of 20 wt%, exist in liquid form <25 °C and transitions to a gel at 37 °C | Sustained drug release, good bioadhesiveness, good biocompatibility | Poor gel durability, weak mechanical strength | [21][22][24][25][26][35] |
| Temperature- Responsive and pH-responsive | p [NIPAAm-co-PAA-co-BA] | Exists in liquid form at room temperature with a pH of 7.4 but transitions into a gel at 37 °C with a pH of 6.8. Able to deliver drug motifs such as bFGF | Gel dissolution and elimination once target is back at normal physiology pH | Increased inflammatory response | [45] |
| Electroconductive | PVV-PANI, PAA, PAMB | Enhanced neural and glial differentiation with electrical stimulation | Drug loading capacity, high bioactivity and cytocompatibility, increased tensile strength and compression | Enhanced cell growth leading to cell death, loss of conductivity, inability to control arrhythmia | [46][47][48][49] |
| Ion-responsive | Salecan + PMAPTA, POG | Binding with negatively charged drugs and stable drug release. Display uniform conductivity and elasticity. | Drug loading capacity, biocompatible, injectable liquid form, controlled biodegradation | Drug release impacted by pH changes, differing affinities to drug binding, and release dependent on charge strength | [4][41][42] |
| Hypoxia-responsive | RAFT, ALOA, PLGA | Increase cell retention, greater oxygen partial pressure capabilities | Excellent biocompatibility, no substantial increase in inflammation | Can trigger ROS burst | [50][51][52][53][54][55] |
| ROS-responsive | CSCl-GSH, TEMPO, NO-RIG, HBPAK, PEDGA | Antioxidant properties effective in facilitating tissue recovery, ROS scavenging, and reduce inflammation | Successfully diminished ROS microenvironment and alleviated hypoxia | Limited retention time to optimize ROS-scavenging capability | [50][51][52][55][56][57][58] |
5. Hypoxia-Responsive Hydrogels
This entry is adapted from the peer-reviewed paper 10.3390/gels8090576
References
- Peng, H.; Ning, X.; Wei, G.; Wang, S.; Dai, G.; Ju, A. The Preparations of Novel Cellulose/Phenylboronic Acid Composite Intelligent Bio-Hydrogel and Its Glucose, PH Responsive Behaviors. Carbohydr. Polym. 2018, 195, 349–355.
- Chen, M.H.; Chung, J.J.; Mealy, J.E.; Zaman, S.; Li, E.C.; Arisi, M.F.; Atluri, P.; Burdick, J.A. Injectable Supramolecular Hydrogel/Microgel Composites for Therapeutic Delivery. Macromol. Biosci. 2018, 19, 1800248.
- Rufaihah, A.J.; Seliktar, D. Hydrogels for Therapeutic Cardiovascular Angiogenesis. Adv. Drug Deliv. Rev. 2016, 95, 31–39.
- Song, X.; Wang, X.; Zhang, J.; Shen, S.; Yin, W.; Ye, G.; Wang, L.; Hou, H.; Qiu, X. A Tunable Self-Healing Ionic Hydrogel with Microscopic Homogeneous Conductivity as a Cardiac Patch for Myocardial Infarction Repair. Biomaterials 2021, 273, 120811.
- Ashley, G.W.; Henise, J.; Reid, R.; Santi, D.V. Hydrogel Drug Delivery System with Predictable and tunable Drug Release and Degradation Rates. Proc. Natl. Acad. Sci. USA 2013, 110, 2318–2323.
- Fan, D.; Tian, Y.; Liu, Z. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Injectable Shear-Thinning Hydrogels for Minimally Invasive Delivery to Infarcted Myocardium to Limit Left Ventricular Remodeling. Available online: https://pubmed-ncbi-nlm-nih-gov.proxy.westernu.edu/27729419/ (accessed on 10 August 2022).
- Shu, Y.; Hao, T.; Yao, F.; Qian, Y.; Wang, Y.; Yang, B.; Li, J.; Wang, C. RoY Peptide-Modified Chitosan-Based Hydrogel to Improve Angiogenesis and Cardiac Repair under Hypoxia. ACS Appl. Mater. Interfaces 2015, 7, 6505–6517.
- Wang, L.L.; Chung, J.J.; Li, E.C.; Uman, S.; Atluri, P.; Burdick, J.A. Injectable and Protease-Degradable Hydrogel for SiRNA Sequestration and Triggered Delivery to the Heart. J. Control. Release 2018, 285, 152–161.
- Aliakbar Ahovan, Z.; Khosravimelal, S.; Eftekhari, B.S.; Mehrabi, S.; Hashemi, A.; Eftekhari, S.; Brouki Milan, P.; Mobaraki, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Thermo-Responsive Chitosan Hydrogel for Healing of Full-Thickness Wounds Infected with XDR Bacteria Isolated from Burn Patients: In Vitro and in Vivo Animal Model. Int. J. Biol. Macromol. 2020, 164, 4475–4486.
- Ter Boo, G.J.; Schmid, T.; Zderic, I.; Nehrbass, D.; Camenisch, K.; Richards, R.G.; Grijpma, D.W.; Moriarty, T.F.; Eglin, D. Local Application of a Gentamicin-Loaded Thermo-Responsive Hydrogel Allows for Fracture Healing upon Clearance of a High Staphylococcus Aureus Load in a Rabbit Model. Eur. Cell Mater. 2018, 35, 151–164.
- Hu, C.-C.; Chiu, Y.-C.; Chaw, J.-R.; Chen, C.-F.; Liu, H.-W. Thermo-Responsive Hydrogel as an Anti-VEGF Drug Delivery System to Inhibit Retinal Angiogenesis in Rex Rabbits. Technol. Health Care 2019, 27, 153–163.
- Liao, H.-T.; Chen, C.-T.; Chen, J.-P. Osteogenic Differentiation and Ectopic Bone Formation of Canine Bone Marrow-Derived Mesenchymal Stem Cells in Injectable Thermo-Responsive Polymer Hydrogel. Tissue Eng. C Methods 2011, 17, 1139–1149.
- Pentlavalli, S.; Chambers, P.; Sathy, B.N.; O’Doherty, M.; Chalanqui, M.; Kelly, D.J.; Haut-Donahue, T.; McCarthy, H.O.; Dunne, N.J. Simple Radical Polymerization of Poly(Alginate-Graft-N-Isopropylacrylamide) Injectable Thermoresponsive Hydrogel with the Potential for Localized and Sustained Delivery of Stem Cells and Bioactive Molecules. Macromol. Biosci. 2017, 17, 1700118.
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lü, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-Based Thermosensitive Hydrogel Containing SWCNTs for Stem Cell Transplantation in Myocardial Repair. Biomaterials 2014, 35, 5679–5688.
- Wang, L.; Li, X.; Sun, T.; Tsou, Y.-H.; Chen, H.; Xu, X. Dual-Functional Dextran-PEG Hydrogel as an Antimicrobial Biomedical Material. Macromol. Biosci. 2018, 18, 1700325.
- Ji, Q.; Zhang, H.; Zhang, X.; Ma, Q.; Teng, L.; Qiu, L. Hydrosoluble Collagen Based Biodegradable Hybrid Hydrogel for Biomedical Scaffold. J. Biomater. Sci. Polym. Ed. 2020, 31, 2199–2219.
- Wang, W.; Deng, L.; Liu, S.; Li, X.; Zhao, X.; Hu, R.; Zhang, J.; Han, H.; Dong, A. Adjustable Degradation and Drug Release of a Thermosensitive Hydrogel Based on a Pendant Cyclic Ether Modified Poly(ε-Caprolactone) and Poly(Ethylene Glycol)Co-Polymer. Acta Biomater. 2012, 8, 3963–3973.
- Wang, P.; Chu, W.; Zhuo, X.; Zhang, Y.; Gou, J.; Ren, T.; He, H.; Yin, T.; Tang, X. Modified PLGA–PEG–PLGA Thermosensitive Hydrogels with Suitable Thermosensitivity and Properties for Use in a Drug Delivery System. J. Mater. Chem. B 2017, 5, 1551–1565.
- Shriky, B.; Kelly, A.; Isreb, M.; Babenko, M.; Mahmoudi, N.; Rogers, S.; Shebanova, O.; Snow, T.; Gough, T. Pluronic F127 Thermosensitive Injectable Smart Hydrogels for Controlled Drug Delivery System Development. J. Colloid Interface Sci. 2020, 565, 119–130.
- Zou, S.; He, Q.; Wang, Q.; Wang, B.; Liu, G.; Zhang, F.; Cheng, X.; Wang, B.; Zhang, L. Injectable Nanosponge-Loaded Pluronic F127 Hydrogel for Pore-Forming Toxin Neutralization. Int. J. Nanomed. 2021, 16, 4239–4250.
- Norouzi, M.; Firouzi, J.; Sodeifi, N.; Ebrahimi, M.; Miller, D.W. Salinomycin-Loaded Injectable Thermosensitive Hydrogels for Glioblastoma Therapy. Int. J. Pharm. 2021, 598, 120316.
- García-Couce, J.; Tomás, M.; Fuentes, G.; Que, I.; Almirall, A.; Cruz, L.J. Chitosan/Pluronic F127 Thermosensitive Hydrogel as an Injectable Dexamethasone Delivery Carrier. Gels 2022, 8, 44.
- Park, K.M.; Lee, S.Y.; Joung, Y.K.; Na, J.S.; Lee, M.C.; Park, K.D. Thermosensitive Chitosan–Pluronic Hydrogel as an Injectable Cell Delivery Carrier for Cartilage Regeneration. Acta Biomater. 2009, 5, 1956–1965.
- Escobar-Chávez, J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.; Quintanar, D.; Ganem, A. Applications of Thermo-Reversible Pluronic F-127 Gels in Pharmaceutical Formulations. J. Pharm. Pharm. Sci. A Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2006, 9, 339–358.
- Kim, Y.C.; Shin, M.D.; Hackett, S.F.; Hsueh, H.T.; Lima E Silva, R.; Date, A.; Han, H.; Kim, B.-J.; Xiao, A.; Kim, Y.; et al. Gelling Hypotonic Polymer Solution for Extended Topical Drug Delivery to the Eye. Nat. Biomed. Eng. 2020, 4, 1053–1062.
- Kozlovskaya, V.; Kharlampieva, E. Self-Assemblies of Thermoresponsive Poly(N-Vinylcaprolactam) Polymers for Applications in Biomedical Field. ACS Appl. Polym. Mater. 2020, 2, 26–39.
- Sala, R.L.; Kwon, M.Y.; Kim, M.; Gullbrand, S.E.; Henning, E.A.; Mauck, R.L.; Camargo, E.R.; Burdick, J.A. Thermosensitive Poly(N-Vinylcaprolactam) Injectable Hydrogels for Cartilage Tissue Engineering. Tissue Eng. A 2017, 23, 935–945.
- Boyaci, T.; Orakdogen, N. Poly(N,N-Dimethylaminoethyl Methacrylate-Co-2-Acrylamido-2-Methyl-Propanosulfonic Acid)/Laponite Nanocomposite Hydrogels and Cryogels with Improved Mechanical Strength and Rapid Dynamic Properties. Appl. Clay Sci. 2016, 121–122, 162–173.
- Zheng, J.Y.; Tan, M.J.; Thoniyot, P.; Loh, X.J. Unusual Thermogelling Behaviour of Poly (PDMAEMA)-Based Polymers Polymerized in Bulk. RSC Adv. 2015, 5, 62314–62318.
- Yu, P.; Xie, J.; Chen, Y.; Liu, J.; Liu, Y.; Bi, B.; Luo, J.; Li, S.; Jiang, X.; Li, J. A Thermo-Sensitive Injectable Hydroxypropyl Chitin Hydrogel for Sustained Salmon Calcitonin Release with Enhanced Osteogenesis and Hypocalcemic Effects. J. Mater. Chem. B 2020, 8, 270–281.
- Li, Z.; Shim, H.; Cho, M.O.; Cho, I.S.; Lee, J.H.; Kang, S.-W.; Kwon, B.; Huh, K.M. Thermo-Sensitive Injectable Glycol Chitosan-Based Hydrogel for Treatment of Degenerative Disc Disease. Carbohydr. Polym. 2018, 184, 342–353.
- Lu, Y.-J.; Lan, Y.-H.; Chuang, C.-C.; Lu, W.-T.; Chan, L.-Y.; Hsu, P.-W.; Chen, J.-P. Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 ShRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy. Int. J. Mol. Sci. 2020, 21, 7111.
- Jung, Y.-S.; Park, W.; Park, H.; Lee, D.-K.; Na, K. Thermo-Sensitive Injectable Hydrogel Based on the Physical Mixing of Hyaluronic Acid and Pluronic F-127 for Sustained NSAID Delivery. Carbohydr. Polym. 2017, 156, 403–408.
- Fan, Z.; Xu, Z.; Niu, H.; Sui, Y.; Li, H.; Ma, J.; Guan, J. Spatiotemporal Delivery of Basic Fibroblast Growth Factor to Directly and Simultaneously Attenuate Cardiac Fibrosis and Promote Cardiac Tissue Vascularization Following Myocardial Infarction. J. Control. Release 2019, 311–312, 233–244.
- Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Kumar, P.; Pillay, V. Rheological and Swelling Behavior of PH Sensitive Hydrogel Particles. APCBEE Procedia 2014, 9, 192–196.
- Garbern, J.C.; Minami, E.; Stayton, P.S.; Murry, C.E. Delivery of Basic Fibroblast Growth Factor with a pH-Responsive, Injectable Hydrogel to Improve Angiogenesis in Infarcted Myocardium. Biomaterials 2011, 32, 2407–2416.
- Rasool, N.; Yasin, T.; Heng, J.Y.Y.; Akhter, Z. Synthesis and Characterization of Novel pH-, Ionic Strength and Temperature- Sensitive Hydrogel for Insulin Delivery. Polymer 2010, 51, 1687–1693.
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators Based on Stimulus-Responsive Hydrogels and Their Emerging Biomedical Applications. NPG Asia Mater. 2019, 11, 64.
- Rodriguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265.
- Wei, W.; Qi, X.; Li, J.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a novel cationic hydrogel base on salecan-g-PMAPTAC. Int. J. Biol. Macromol. 2017, 101, 474–480.
- Lanzalaco, S.; Armelin, E. Poly(N-Isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36.
- Schmaljohann, D. Thermo- and pH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670.
- Matsumura, Y.; Zhu, Y.; Jiang, H.; D’Amore, A.; Luketich, S.K.; Charwat, V.; Yoshizumi, T.; Sato, H.; Yang, B.; Uchibori, T.; et al. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289.
- Zhang, C.; Hsieh, M.-H.; Wu, S.-Y.; Li, S.-H.; Wu, J.; Liu, S.-M.; Wei, H.-J.; Weisel, R.D.; Sung, H.-W.; Li, R.-K. A self-doping conductive polymer hydrogel that can restore electrical impulse propagation at myocardial infarct to prevent cardiac arrhythmia and preserve ventricular function. Biomaterials 2020, 231, 119672.
- Niu, H.; Li, C.; Guan, Y.; Dang, Y.; Li, X.; Fan, Z.; Shen, J.; Ma, L.; Guan, J. High oxygen preservation hydrogels to augment cell survival under hypoxic condition. Acta Biomater. 2020, 105, 56–67.
- Ruparelia, N.; Chai, J.T.; Fisher, E.A.; Choudhury, R.P. Inflammatory processes in cardiovascular disease: A route to targeted therapies. Nat. Rev. Cardiol. 2017, 14, 133–144.
- Zhao, B.; He, J.; Wang, F.; Xing, R.; Sun, B.; Zhou, Y. Polyacrylamide-Sodium Alginate Hydrogel Releasing Oxygen and Vitamin C Promotes Bone Regeneration in Rat Skull Defects. Front. Mater. 2021, 8, 469.
- Zhu, Y.; Matsumura, Y.; Velayutham, M.; Foley, L.M.; Hitchens, T.K.; Wagner, W.R. Reactive oxygen species scavenging with a biodegradable, thermally responsive hydrogel compatible with soft tissue injection. Biomaterials 2018, 177, 98–112.
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable Fullerenol/Alginate Hydrogel for Suppression of Oxidative Stress Damage in Brown Adipose-Derived Stem Cells and Cardiac Repair. ACS Nano 2017, 11, 5474–5488.
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel—Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152.
- Camci-Unal, G.; Annabi, N.; Dokmeci, M.R.; Liao, R.; Khademhosseini, A. Hydrogels for Cardiac Tissue Engineering. NPG Asia Mater. 2014, 6, e99.
- Thi, P.L.; Lee, Y.; Tran, D.L.; Thi, T.T.H.; Park, K.M.; Park, K.D. Calcium peroxide-mediated in situ formation of multifunctional hydrogels with enhanced mesenchymal stem cell behaviors and antibacterial properties. J. Mater. Chem. B 2020, 8, 11033–11043.
- Li, Z.; Guo, X.; Guan, J. An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials 2012, 33, 5914–5923.
- Shiekh, P.A.; Singh, A.; Kumar, A. Oxygen-Releasing Antioxidant Cryogel Scaffolds with Sustained Oxygen Delivery for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 18458–18469.
- Komeri, R.; Thankam, F.G.; Muthu, J. Free Radical Scavenging Injectable Hydrogels for Regenerative Therapy. Mater. Sci. Eng. C 2017, 71, 100–110.
- Finosh, G.T.; Jayabalan, M. Reactive Oxygen Species—Control and Management Using Amphiphilic Biosynthetic Hydrogels for Cardiac Applications. Adv. Biosci. Biotechnol. 2013, 4, 1134–1146.
- Fan, Z.; Xu, Z.; Niu, H.; Gao, N.; Guan, Y.; Li, C.; Dang, Y.; Cui, X.; Liu, X.L.; Duan, Y.; et al. An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci. Rep. 2018, 8, 1371.
- Alemdar, N.; Leijten, J.; Camci-Unal, G.; Hjortnaes, J.; Ribas, J.; Paul, A.; Mostafalu, P.; Gaharwar, A.K.; Qiu, Y.; Sonkusale, S.; et al. Oxygen-Generating Photo-Cross-Linkable Hydrogels Support Cardiac Progenitor Cell Survival by Reducing Hypoxia-Induced Necrosis. ACS Biomater. Sci. Eng. 2017, 3, 1964–1971.
