Drought stress is a major abiotic stress that significantly affects agricultural productivity every year as the plants undergo several morphological, biochemical, and physiological modifications, such as repressed root and shoot growth, reduced photosynthesis and transpiration rate, excessive production of reactive oxygen species (ROS), osmotic adjustments, and modified leaf senescence regulating and stress signaling pathways. Such modifications may permanently damage the plants; therefore, mitigation strategies must be developed. The use of drought-resistant crop cultivars is more expensive and labor-intensive with few advantages. However, exploiting plant growth promoting rhizobacteria (PGPR) is a proven alternative with numerous direct and indirect advantages.
- antioxidants
- drought
- induced systemic tolerance
- phytohormones
- plant growth promoting rhizobacteria
1. Introduction
2. Plant Growth Promoting Rhizobacteria Mediated Drought Stress Tolerance in Plants
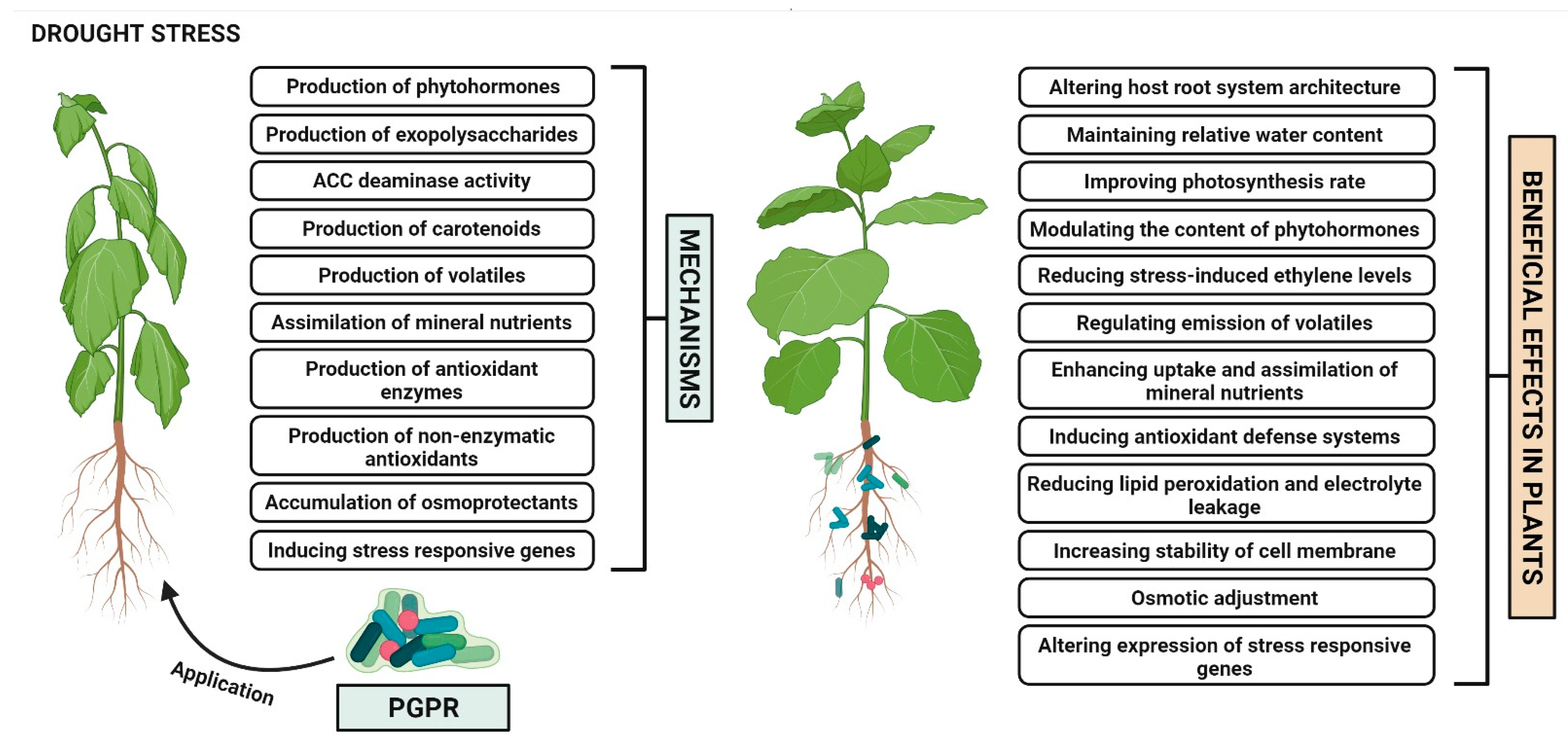
2.1. Alteration of Host Root System Architecture
2.2. Maintenance of Relative Water Content
2.3. Improvement of Photosynthesis Rate
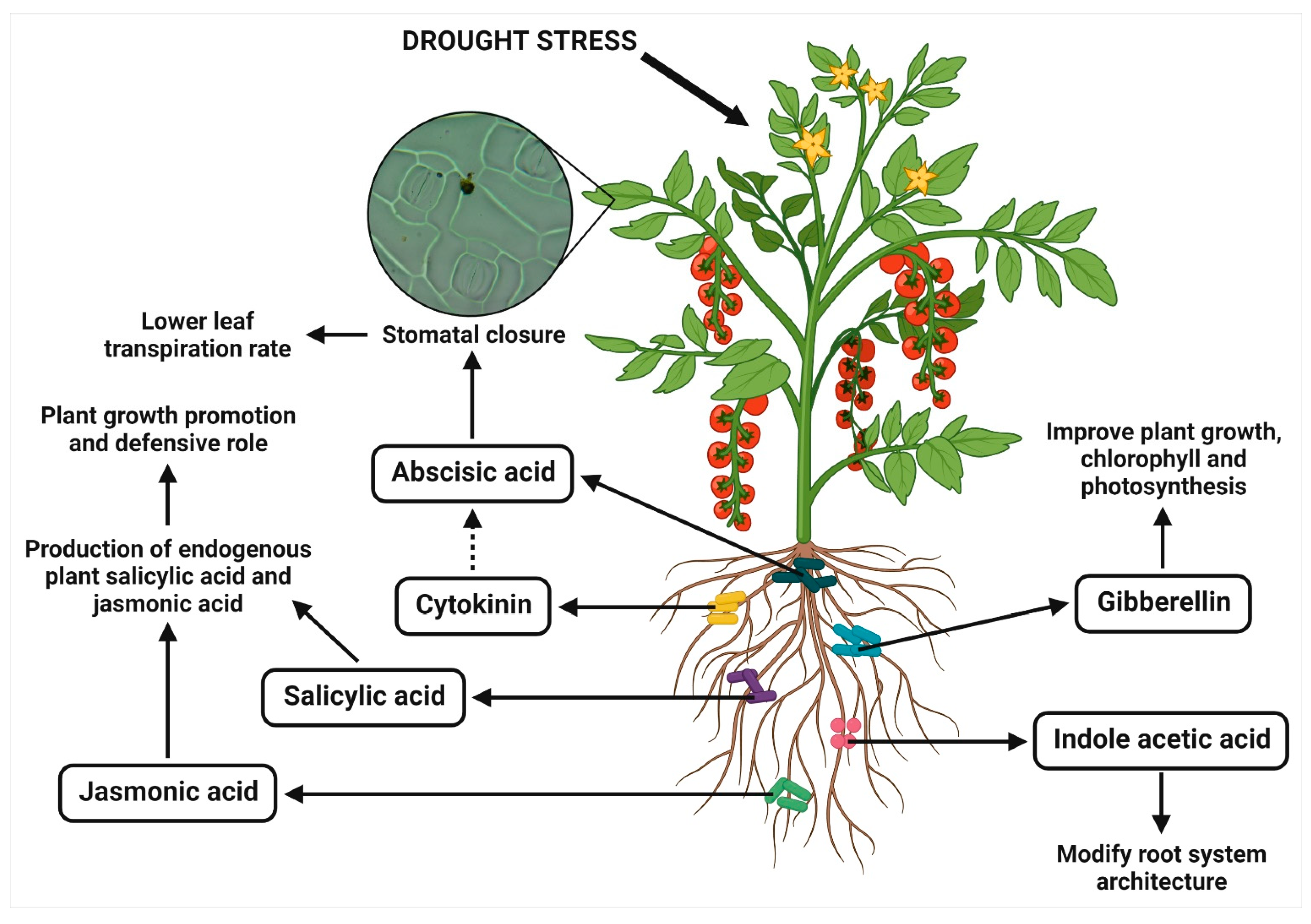
2.4. Production of Phytohormones
| PGPR Strains | Plant Species | Mechanisms | Beneficial Effects | Reference |
|---|---|---|---|---|
| Bacillus megaterium and Pseudomonas putida | Trifolium repens | IAA production | Enhanced the water content, root and shoot biomass | [16] |
| Bacillus amyloliquefaciens, B. muralis, B. pumilus, B. simplex, B. thuringiensis, Enterobacter aerogenes, Moraxella pluranimalium, and P. stutzeri |
T. aestivum | IAA production | Significantly improved the shoot length, spike length, seed weight, tillers and number of spikelets, and increased the peroxidase, acid phosphatase and proline content in plants | [40] |
| Bacillus sp. and Enterobacter sp. | T. aestivum and Z. mays | IAA and salicylic acid production | Displayed the root system architecture alteration viz., increased the number of root tips, root surface area, root length and root branching | [18] |
| P. aeruginosa | Vigna radiata | IAA production | Enhanced the shoot length, number of grains, pod/plant, total yield, 100 seed weight and 100 seed straw weight, improved the photosynthetic activity, membrane stability, relative water content and antioxidant efficacy | [41] |
| B. subtilis | Lactuca sativa | Cytokinin production | Increased the shoot cytokinins, stimulated the shoot mass accumulation and shortened roots, decreased stomatal conductance and root/shoot ratios | [42] |
| B. subtilis | Platycladus orientalis | Cytokinin production | Showed the higher leaf relative water content and water potential, increased the plant root exudates (like amino acids, sugars and organic acids) and stomatal conductance, and elevated the levels of cytokinins in shoot | [43] |
| A. calcoaceticus | S. williamsii | Cytokinin production | Increased the photosynthetic rate, decreased the stomatal conductance and intracellular CO2 concentration | [44] |
| P. putida | Glycine max | Gibberellin production | Increased the shoot length and fresh weight, higher chlorophyll content, lower levels of ABA and salicylic acid, higher jasmonic acid level, reduced sodium content, increased phosphate content and modulated the antioxidants by decreasing radical scavenging activity, SOD and flavonoids | [45] |
| B. licheniformis and P. fluorescens | Vitis vinifera | ABA production | Increased the plant ABA levels, diminished the water loss rate and incremented the synthesis of defense-related terpenes | [46] |
| B. marisflavi | Brassica juncea | ABA analogue/xanthoxin production | Delayed the drooping points of plants and higher drought stress tolerance index, induced the stomatal closure, inhibited the seed germination, and decreased the gibberellic acid induced α-amylase activity | [29] |
This entry is adapted from the peer-reviewed paper 10.3390/antiox11091763
References
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86.
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259.
- Saleem, A.R.; Brunetti, C.; Khalid, A.; Rocca, G.D.; Raio, A.; Emiliani, G.; Carlo, A.D.; Mahmood, T.; Centritto, M. Drought response of Mucuna pruriens (L.) DC. inoculated with ACC deaminase and IAA producing rhizobacteria. PLoS ONE 2018, 13, e0191218.
- Jabborova, D.; Annapurna, K.; Fayzullaeva, M.; Sulaymonov, K.; Kadirova, D.; Jabbarov, Z.; Sayyed, R.Z. Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.). Ann. Phytomed. 2020, 9, 116–121.
- Murali, M.; Gowtham, H.G.; Brijesh Singh, S.; Shilpa, N.; Aiyaz, M.; Niranjana, S.R.; Amruthesh, K.N. Bio-prospecting of ACC deaminase producing rhizobacteria towards sustainable agriculture: A special emphasis on abiotic stress in plants. Appl. Soil Ecol. 2021, 168, 104142.
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149.
- Sayyed, R.Z.; Patel, P.R. Biocontrol potential of siderophore producing heavy metal resistant Alcaligenes sp. and Pseudomonas aeruginosa RZS3 vis-à-vis organophosphorus fungicide. Indian J. Microbiol. 2011, 51, 266–272.
- Ferreira, M.J.; Silva, H.; Cunha, A. Siderophore-producing rhizobacteria as a promising tool for empowering plants to cope with iron limitation in saline soils: A Review. Pedosphere 2019, 29, 409–420.
- Shaikh, S.S.; Wani, S.J.; Sayyed, R.Z. Statistical-based optimization and scale-up of siderophore production process on laboratory bioreactor. 3 Biotech 2016, 6, 69.
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 580024.
- Arzanesh, M.H.; Alikhani, H.A.; Khavazi, K.; Rahimian, H.A.; Miransari, M. Wheat (Triticum aestivum L.) growth enhancement by Azospirillum sp. under drought stress. World J. Microbiol. Biotechnol. 2011, 27, 197–205.
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Modified chrome azurol S method for detection and estimation of siderophores having affinity for metal ions other than iron. Environ. Sustain. 2018, 1, 81–87.
- Sayyed, R.Z.; Seifi, S.; Patel, P.R.; Shaikh, S.S.; Jadhav, H.P.; El Enshasy, H. Siderophore production in groundnut rhizosphere isolate, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Environ. Sustain. 2019, 2, 117–124.
- Cohen, A.C.; Bottini, R.; Pontin, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90.
- Jabborova, D.; Wirth, S.; Kannepalli, A.; Narimanov, A.; Desouky, S.; Davranov, K.; Sayyed, R.Z.; El Enshasy, H.; Malek, R.A.; Syed, A.; et al. Co-inoculation of rhizobacteria and biochar application improves growth and nutrients in Soybean and enriches soil nutrients and enzymes. Agronomy 2020, 10, 1142.
- Marulanda, A.; Barea, J.M.; Azcón, R. Stimulation of plant growth and drought tolerance by native microorganisms (AM fungi and bacteria) from dry environments: Mechanisms related to bacterial effectiveness. J. Plant Growth Regul. 2009, 28, 115–124.
- Huang, X.F.; Zhou, D.; Lapsansky, E.R.; Reardon, K.F.; Guo, J.; Andales, M.J.; Vivanco, J.M.; Manter, D.K. Mitsuaria sp. and Burkholderia sp. from Arabidopsis rhizosphere enhance drought tolerance in Arabidopsis thaliana and maize (Zea mays L.). Plant Soil 2017, 419, 523–539.
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microbiol. 2019, 10, 2106.
- Silva, E.R.; Zoz, J.; Oliveira, C.E.S.; Zuffo, A.M.; Steiner, F.; Zoz, T.; Vendruscolo, E.P. Can co-inoculation of Bradyrhizobium and azospirillum alleviate adverse effects of drought stress on soybean (Glycine max L. Merrill.)? Arch. Microbiol. 2019, 201, 325–335.
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; LuhSuriani, N.; Herlambang, S. Production, purification, and characterization of Bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in Sesame. Sustainability 2021, 13, 5394.
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought stress in plants: An over view. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33.
- Castillo, P.; Escalante, M.; Gallardo, M.; Alemano, S.; Abdala, G. Effects of bacterial single inoculation and co-inoculation on growth and phytohormone production of sunflower seedlings under water stress. Acta Physiol. Plant. 2013, 35, 2299–2309.
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 105, 109–125.
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032.
- García, J.E.; Maroniche, G.; Creus, C.; Suárez-Rodríguez, R.; Ramirez-Trujillo, J.A.; Groppa, M.D. In Vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2017, 202, 21–29.
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140.
- Khan, N.; Bano, A. Exopolysaccharide producing rhizobacteria and their impact on growth and drought tolerance of wheat grown under rainfed conditions. PLoS ONE 2019, 14, e0222302.
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368.
- Gowtham, H.G.; Duraivadivel, P.; Ayusman, S.; Sayani, D.; Gholap, S.L.; Niranjana, S.R.; Hariprasad, P. ABA analogue produced by Bacillus marisflavi modulates the physiological response of Brassica juncea L. under drought stress. Appl. Soil Ecol. 2021, 159, 103845.
- Duan, B.; Li, L.; Chen, G.; Su-Zhou, C.; Li, Y.; Merkeryan, H.; Liu, W.; Liu, X. 1-Aminocyclopropane-1-carboxylate deaminase-producing plant growth-promoting rhizobacteria improve drought stress tolerance in Grapevine (Vitis vinifera L.). Front. Plant Sci. 2021, 12, 706990.
- Nadeem, S.M.; Ahmad, M.; Tufail, M.A.; Asghar, H.N.; Nazli, F.; Zahir, Z.A. Appraising the potential of EPS-producing rhizobacteria with ACC-deaminase activity to improve growth and physiology of maize under drought stress. Physiol. Plant. 2020, 172, 463–476.
- Timmusk, S.; Abd El-Daim, I.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L.; Nevo, E.; Seisenbaeva, G.; Stenström, E.; Niinemets, Ü. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, e96086.
- Martins, S.J.; Rocha, G.A.; de Melo, H.C.; Georg, R.D.C.; Ulhôa, C.J.; Dianese, É.D.C.; Oshiquiri, L.H.; da Cunha, M.G.; da Rocha, M.R.; de Araújo, L.G.; et al. Plant-associated bacteria mitigate drought stress in soybean. Environ. Sci. Pollut. Res. 2018, 25, 13676–13686.
- Liu, F.; Ma, H.; Peng, L.; Du, Z.; Ma, B.; Liu, X. Effect of the inoculation of plant growth-promoting rhizobacteria on the photosynthetic characteristics of Sambucus williamsii Hance container seedlings under drought stress. AMB Expr. 2019, 9, 169.
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104.
- Hariprasad, P.; Gowtham, H.G.; Gourav, C. Beneficial plant-associated bacteria modulate host hormonal system enhancing plant resistance toward abiotic stress. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Kidlington, UK, 2021; pp. 113–151.
- Khan, I.; Awan, S.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. Effects of 24-epibrassinolide on plant growth, antioxidants defense system, and endogenous hormones in two wheat varieties under drought stress. Physiol. Plant. 2021, 172, 696–706.
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598.
- Khan, N.; Ali, S.; Shahid, M.A.; Mustafa, A.; Sayyed, R.Z.; Curá, J.A. Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 2021, 10, 1551.
- Raheem, A.; Shaposhnikov, A.; Belimov, A.A.; Dodd, I.C.; Ali, B. Auxin production by rhizobacteria was associated with improved yield of wheat (Triticum aestivum L.) under drought stress. Arch. Agron. Soil Sci. 2018, 64, 574–587.
- Uzma, M.; Iqbal, A.; Hasnain, S. Drought tolerance induction and growth promotion by indole acetic acid producing Pseudomonas aeruginosa in Vigna radiate. PLoS ONE 2022, 17, e0262932.
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin producing bacteria enhance plant growth in drying soil. Plant Soil 2007, 292, 305–315.
- Liu, F.; Xing, S.; Ma, H.; Du, Z.; Ma, B. Cytokinin-producing, plant growth-promoting rhizobacteria that confer resistance to drought stress in Platycladusorientalis container seedlings. Appl. Microbiol. Biotechnol. 2013, 97, 9155–9164.
- Gowtham, H.G.; Murali, M.; Brijesh Singh, S.; Lakshmeesha, T.R.; Murthy, K.; Amruthesh, K.N.; Niranjana, S.R. Plant growth promoting rhizobacteria- Bacillus amyloliquefaciens improves plant growth and induces resistance in chilli against anthracnose disease. Biol. Control 2018, 126, 209–217.
- Kang, S.M.; Radhakrishnan, R.; Khan, A.L.; Kim, M.J.; Park, J.M.; Kim, B.R.; Shin, D.H.; Lee, I.J. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol. Biochem. 2014, 84, 115–124.
- Salomon, M.V.; Bottini, R.; de Souza Filho, G.A.; Cohen, A.C.; Moreno, D.; Gil, M.; Piccoli, P. Bacteria isolated from roots and rhizosphere of Vitis vinifera retard water losses, induce abscisic acid accumulation and synthesis of defense-related terpenes in in vitro cultured grapevine. Physiol. Plant. 2014, 151, 359–374.
