Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Immunology
The alveolar epithelium serves as a barrier between the body and the external environment. To maintain efficient gas exchange, the alveolar epithelium has evolved to withstand and rapidly respond to an assortment of inhaled, injury-inducing stimuli. Alveolar damage can lead to loss of alveolar fluid barrier function and exuberant, non-resolving inflammation that manifests clinically as acute respiratory distress syndrome (ARDS).
- ARDS
- alveolar epithelium
- lung repair
- lung regeneration
1. Alveolar Epithelial Structure
Structural features of the alveolar epithelium include the alveolar epithelial cell monolayer and alveolar lining layer (Figure 1). The cellular monolayer is a mosaic of squamous alveolar type 1 (AT1) and cuboidal alveolar type 2 (AT2) cells whose basement membranes abut the alveolar interstitium. Opposite the interstitium lie the basement membranes and cells of the pulmonary microvascular endothelium. Together, the alveolar epithelium, alveolar interstitium, and microvascular endothelium form the alveolar barrier.
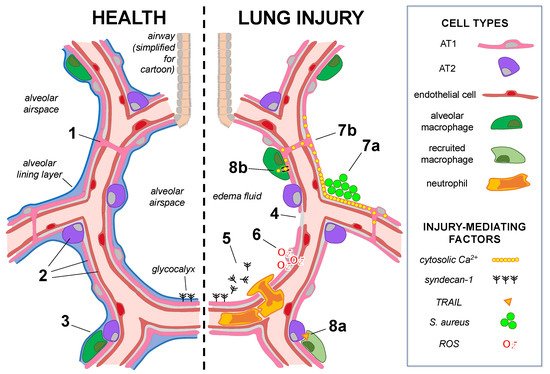
Figure 1. Mechanisms of alveolar epithelial health and lung injury. The cartoon shows alveoli under baseline conditions (left) in which alveolar epithelial type 1 (AT1) and type 2 (AT2) cells underlie the alveolar lining layer. The glycocalyx extends from the epithelial surface. (Note: the glycocalyx size is exaggerated.) AT1 cells span multiple alveoli through the pores of Kohn in human lungs (1), and AT1, AT2, and microvascular endothelial cells (2) are heterogeneous groups comprised of subpopulations. AT2-cell-derived GM-CSF (3) maintains the lung resident alveolar macrophage population in healthy adult lungs. In lung injury (right), alveolar epithelial cell damage, junctional and Na,K-ATPase protein loss (4), and surfactant dysfunction lead to edema fluid accumulation in airspaces. (5) Alveolar responses to injury: Glycocalyx shedding promotes airspace neutrophil recruitment and activation. (6) Epithelial–endothelial crosstalk: ROS release from the injured alveolar epithelium causes mitochondrial depolarization and cytoskeletal destabilization in the epithelium-adjacent endothelium. (7a,b) Alveolar epithelial cell–cell communication: (7a) Inhaled S. aureus form microaggregates in structural alveolar niches, where localized increases in cytosolic Ca2+ spread (7b) through alveolar epithelial gap junctions to induce widespread alveolar edema. (8a,b) Reciprocal signaling between the alveolar epithelium and immune cells: (8a) Interferon alpha secretion by influenza-infected AT2 cells induces secretion of TNF-related apoptosis-induced ligand (TRAIL) by recruited macrophages, inducing signaling to the uninfected alveolar epithelium, which leads to epithelial loss of Na,K-ATPase protein. (8b) Alveolar macrophages directly interact with the alveolar epithelium via gap junctions to communicate anti-inflammatory cytosolic Ca2+ signals, limiting lung injury.
The alveolar barrier facilitates gas exchange between air and blood compartments of the lung. As reviewed by Weibel [1], the body’s metabolic needs require that gas exchange be efficient; thus, the alveolar barrier must be thin and extensive. AT1 cells are well-suited to gas exchange owing to their attenuated cytoplasmic leaflets, which are in many places less than 0.2 µm thick and spread broadly across adjacent alveoli to span an average surface area of 5000 µm2 per cell in human lungs [1,2,3,4]. AT2 cells have a smaller apical surface area of approximately 200 µm2 per cell but are more numerous [3]. Across the mammalian spectrum, increased body mass correlates primarily with increased AT1 and AT2 cell number [5], resulting in an average alveolar epithelial surface area of more than 100 m2 in adult human lungs [2,6]—a remarkable size.
The alveolar lining layer is located between the apical alveolar epithelial surface and alveolar airspace. It is thin and continuous but pools at alveolar curvatures, where alveolar septa converge [7]. The lining layer consists of two phases: an aqueous hypophase in contact with the alveolar epithelium and a surfactant film that forms the air–liquid interface [8,9]. The hypophase contains reserve surfactant material [8,9] and the alveolar epithelial glycocalyx—a multilayered structure of proteoglycans and glycoproteins anchored to apical membranes of alveolar epithelial cells (reviewed in [10]).
2. Alveolar Epithelial Homeostatic Function
Homeostatic functions of the alveolar epithelium maintain the integrity of the apical lung surface and ensure that the alveolar airspaces remain air-filled for gas exchange. Maintenance of air-filled alveoli requires that the alveolar epithelium secrete surfactant and hypophase liquid and resist mechanical forces, including microvascular hydrostatic pressure, that tend to drive fluid from microvessels into airspaces (reviewed in [1]). Maintenance of apical lung surface integrity requires that the alveolar epithelium defend itself against inhaled pathogens and undergo basal cell turnover as needed.
The alveolar epithelial barrier restricts the paracellular passage of molecules, ions, and liquid into alveolar airspaces [11,12,13,14]. Epithelial barrier permeability is determined primarily by the expression and regulation of tight junctional proteins, particularly those of the claudin family [12,15,16,17]. Adherens junction proteins and ion transport-independent functions of the epithelial Na,K-ATPase contribute to barrier function by promoting epithelial and junctional protein integrity [18,19,20,21] (reviewed in [22,23,24]). Under basal conditions, therefore, junctional and Na,K-ATPase proteins separate the alveolar epithelial apical and basolateral membranes to establish epithelial polarity and regulate the paracellular movement of solutes and liquid, thereby preventing spontaneous edema formation and maintaining airspaces for gas exchange. However, when excess fluid is introduced into alveolar airspaces, apical epithelial Na+ channels (ENaC) and the basolateral Na,K-ATPase drive transepithelial Na+ uptake [25,26,27,28,29,30], generating electrochemical and osmotic gradients that induce absorption of Cl− and water. The excess liquid is likely resorbed by the lymphatic system via the interstitium [31,32].
The alveolar lining layer is secreted by the alveolar epithelium and is essential to the gas exchange function of alveoli. Alveolar surfactant is a heterogeneous mixture of lipids and proteins that lowers surface tension at the air–liquid interface to maintain airspace patency [33,34,35] (reviewed in [36,37]). Surfactant is stored in AT2 cell lamellar bodies and secreted into the hypophase in response to lung inflation and other stimuli before it is taken up, processed, and re-secreted by AT2 cells [38] (reviewed in [39]). The hypophase provides a medium for surfactant system components and is generated continuously by chloride-dependent liquid secretion into alveolar spaces through the alveolar epithelial cystic fibrosis transmembrane conductance regulator (CFTR) protein [14,40,41,42].
The alveolar epithelium defends alveoli against cellular damage induced by inhaled particles and pathogens. AT2 cell secretion of granulocyte–macrophage colony-stimulating factor (GM-CSF) maintains the steady-state population of lung resident AMs [43], cells whose surveillance and phagocytosis functions are critical to alveolar defense. AT2 cell-derived surfactant proteins (SP)-A and SP-D promote AM opsonization and phagocytosis of pathogens and directly inhibit growth of Gram-negative bacteria and fungi [44,45]. Alveolar epithelial hypophase secretion may facilitate AM function by retaining and perhaps opsonizing inhaled particles [46]. In addition, hypophase secretion contributes directly to alveolar defense through pH-mediated regulation of SP-A and SP-D activity [44,47] and generation of alveolar liquid flow that convectively transports particles out of alveoli [40].
To maintain gas exchange over a lifetime, the alveolar epithelium must undergo homeostatic cell turnover. Recent research sheds new light on alveolar epithelial turnover rates and mechanisms. In mice, lineage tracing studies indicate homeostatic alveolar epithelial turnover is a slow process [48,49]. The turnover mechanism may depend on juxtacrine interactions between alveolar interstitial fibroblasts and AT2 cells [48], which serve as stem cells for the alveolar epithelium [50]. Notably, a rare AT2 cell subpopulation appears to have higher replication capacity under baseline conditions [51]. Cells of this subpopulation tend to be located near pulmonary microvessels [51], but their role in homeostasis, the significance of their location, and the extent to which the cells communicate with microvascular endothelia or pericapillary cells remains unknown. The recent finding that the alveolar capillary endothelium is also comprised of subpopulations [52] raises the intriguing possibility that specialized cells of the alveolar epithelium and adjacent microvascular endothelium interact to maintain alveolar health.
This entry is adapted from the peer-reviewed paper 10.3390/biom12091273
This entry is offline, you can click here to edit this entry!
