Overall, changes in diet or nutritional status and deficiencies in certain nutrients, such as vitamin D, can increase the risk of developing asthma. Vitamin D deficiency can cause linear growth disorders such as stunting in children, with lower levels of 25(OH)D found in underweight and stunted children. Stunted children show a decreased lean body mass, which affects lung growth and function. Low leptin levels during undernutrition cause a Th1–Th2 imbalance toward Th2, resulting in increased interleukin (IL)-4 cytokine production and total immunoglobulin E (IgE). Studies in stunted underweight children have also found an increase in the proportion of the total number of B cells with low-affinity IgE receptors (CD23+). CD23+ plays an important role in allergen presentation that is facilitated by IgE to T cells and strongly activates allergen-specific T cells and the secretion of Th2-driving cytokines. Stunted children present with low vitamin D and leptin levels, impaired lung growth, decreased lung function, and increased IL-4 and CD23+ levels. All of these factors may be considered consequential in asthma in stunted children.
1. Vitamin D
Among the numerous functions of vitamin D as a hormone, the classic functions are mineral balance and bone maintenance while it also act as a micronutrient and immunomodulator. As an immunomodulator, 1,25(OH)
2D
3 suppresses Th1 cell activation, modulates Th2 cells, and increases Treg cell activity [
27]. Although asthma is a known Th2-mediated disease, the impact of vitamin D on Th2 cells reportedly differs. The impact of 1,25(OH)
2D
3 on Th2 cells remains controversial, with evidence suggesting that 1,25(OH)
2D
3 can inhibit IL-4 transcription but can also upregulate IL-4 in mouse T cells [
34,
35].
Vitamin D alters the balance of Th1–Th2 cytokine toward Th2, resulting in reduced secretion of Th1 cytokines IL-2 and IFN-γ and an increase in the Th2 cytokine IL-4. In contrast, in CD4+ as well as CD8 human cord blood cells, vitamin D inhibits IL-12-generated IFN-γ production and suppresses IL-4 and IL-4-induced expression of IL-13 [
20]. Staeva-Vieira reported that during in vitro polarization, 1,25(OH)
2D
3 inhibited Th1 (IFN-γ) and Th2 (IL-4) cytokines on naïve CD62 ligand
+CD4
+ T cells [
34]. The contradictory effect of vitamin D on Th1–Th2 dominance may be due to the effect of vitamin D on T-regulatory (Treg) cells. Vitamin D has been shown to increase Treg cell induction [
20].
2. Leptin
Various studies have shown that malnourished children present with low levels of growth hormone, leptin, and prolactin, all of which promote thymic growth and function [
38]. Leptin is a hormone/cytokine derived from adipocytes and links the nutritional conditions with neuroendocrine and immune systems [
8]. Leptin has direct and indirect effects on the number and function of T cells, increasing the number of cells and the Th1 and Th17 cytokines while also inhibiting the production of Th2 cytokines and Treg proliferation [
6]. Adipocyte-derived leptin secretion has been associated with normal regulation of metabolic function and a balance between Th1 and Th2/Treg cells that causes the suppression of immune and autoimmune responses [
8]..
3. Interleukin (IL)-4
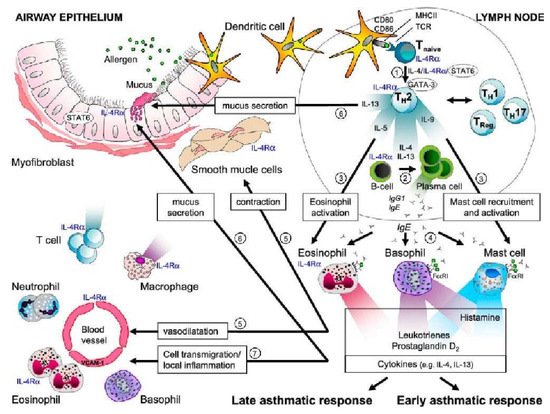
IL-4 is produced by Th2 cells, mast cells, basophils, eosinophils, and the alveolar macrophages [
39]. IL-4 is a pleiotropic cytokine that binds to the IL-4 receptor expressed by T lymphocytes, B lymphocytes, mononuclear phagocytes, eosinophils, pulmonary fibroblasts, endothelial cells, bronchial epithelial cells, and smooth muscle cells. IL-4 is involved in the different mechanisms leading to allergic airway disease. Transcription factors are activated by the IL-4/IL-13 signaling cascade (
Figure 2) [
39].
Figure 2. Role of IL-4 in asthma and allergy. Arrow 1 shows the role of IL-4 in the differentiation of T cells into Th2 cells. Arrow 2 shows the class-switching isotype of B cells that synthesize IgE, which is mediated by IL-4 and IL-13. Arrow 3 shows the effect of IL-5 on eosinophils and that of IL-9 on mast cell function. Arrow 4 indicates that IgE produced by plasma cells binds to the IgE receptors on mast cells, basophils, and eosinophils. Upon allergen exposure, IgE crosslinking causes an immediate release of histamine, leukotrienes, and prostaglandins (after a few minutes) and the production of other cytokines (after a few hours). Arrow 5 shows that histamine, leukotrienes, and prostaglandins induce smooth muscle contractions and vasodilation. Arrow 6 indicates that cytokine IL-13, together with IL-4, causes mucus secretion and goblet cell hyperplasia. Arrow 7 shows that IL-4 and IL-13 contribute to the recruitment of inflammatory cells by upregulating the expression of VCAM-1, which facilitates the transmigration of eosinophils, T cells, monocytes, and basophils in response to chemokines produced by mast cells (the late asthma response) [
39]. AHR, airway hyperresponsiveness; FcɛRI, Fc epsilon RI, high-affinity receptor for the Fc region of immunoglobulin E (IgE); IL-4Ra, interleukin-4 receptor a; Stat6, signal transducer and activator of transcription 6. Reprinted with permission from Ref. [
39]. Copyright 2022 American Thoracic Society.IL-4 is the main cytokine in the sequential development of allergic inflammatory reactions. It mediates an important proinflammatory function in asthma by inducing IgE isotype switch and IgE secretion via B lymphocytes and vascular cell adhesion molecule-1 (VCAM-1) expression, increasing eosinophil transmigration through the endothelium, mucus production, and Th2 lymphocyte differentiation and promoting cytokine release. IL-4 increases IgE-mediated immune responses by regulating IgE receptors, namely the low-affinity IgE receptor (FcεRII or CD23+) on B lymphocytes, mononuclear phagocytic cells, and the high-affinity IgE receptor (FcεRI) on mast cells and basophils. IL-4 contributes to airway obstruction in asthma by inducing mucin gene expression and increasing mucus secretion and other inflammatory cytokines from fibroblasts, which contribute to the inflammatory process and pulmonary remodeling [
39].
4. CD23+
A low-affinity receptor for IgE, namely FcεRII or CD23+, presents an important role in regulating IgE responses. The regions of CD23+ responsible for its interaction with several ligands, which include IgE, CD21, major histocompatibility complex class II, and integrins, were discovered [
40].
The interaction between IgE and the CD23+ remains unclear and has not been comprehended completely compared to the interaction between IgE and the high-affinity IgE receptor (FcεRI), even though CD23+ plays many significant roles in the allergic inflammatory process. One of the important roles of CD23+ is presenting allergen to T cells, which are facilitated by IgE. IgE-facilitated allergen presentation potentially activates allergen-specific T cells and secretes Th2-driving cytokine [
41].
Overall, the expression of CD23+ surface density on B cells among allergic patients will increase in the presence of high levels of allergen-specific IgE. This leads to enhance IgE-facilitated allergen presentation and allergen-specific T-cell activation [
41]. T-cell-mediated allergic inflammation induced by IgE-facilitated allergen presentation might be controlled by CD23+ surface density on B cells and allergen-specific T-cell activation [
42]. The role of CD23+ in allergic airway disease is explained in
Figure 3 [
43].
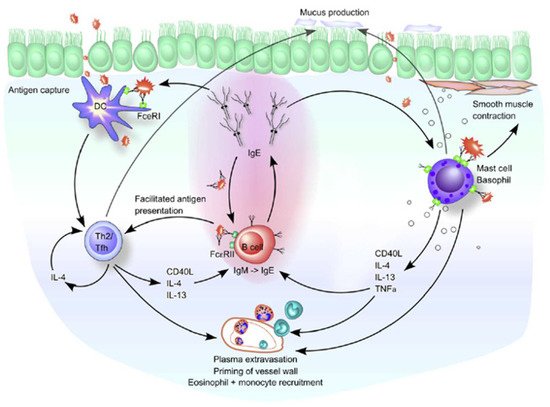
Figure 3. Role of CD23+/FcεRII and IgE in allergic airway diseases. DCs initially take up inhaled antigens in an atopic environment and result in a predominant induction of Th2 cells. Th2 cells potentially induce IgE-producing B cells through the production of IL-4 and IL-13. The resulting IgE then binds to its high-affinity (FcεRI) and low-affinity (CD23+/FcεRII) receptors on the airway mucosa cells. The binding of FcεRI-bound IgE to the allergen on mast cells and basophils causes degranulation and release of inflammatory mediators and cytokines, leading to immediate hypersensitivity symptoms. The late-phase response begins when the released Th2 cytokines (IL-4, IL-5, and IL-13) recruit and activate the inflammatory cells, such as monocytes and eosinophils. IL-4 and IL-13 also cause excessive mucus production by goblet cells. Elevated local IgE levels induce FcεRI expression on DCs and stabilize CD23+/FcεRII on B cells. Therefore, in a Th2-skewing environment, these cell types can take up allergen-IgE complexes and present the allergen to T cells. For B cells bearing CD23+/FcεRII, this is called facilitated antigen presentation because it occurs in a noncognate manner. Reprinted with permission from Ref. [
43]. Copyright 2022 Journal of Allergy and Clinical Immunology.Chary et al. reported that children with asthma had a higher proportion of B cells with CD23+ expression than controls [
44]. CD23+ expression of lymphocytes among children with asthma (due to allergic response) increases and is positively associated with serum IgE levels [
40]. Excessive expression of CD23+ in patients with extrinsic asthma results from excessive IL-4 production by allergen-specific T cells [
45]. The expression of CD23+ has been associated with allergic diseases. Anti-CD23 therapy may be a promising candidate therapy for allergic diseases in the future. Anti-CD23 might induce tolerance, block antigen presentation, suppress T-cell differentiation, and diminish APC activation in allergic reactions [
46].
Studies on stunted underweight children also found an increase in the proportion of the total number of B cells with CD23+, impaired T-cell responses, and increased levels of total IgE and IL-4 [
10]. Theoretically, the important roles of CD23+ is IgE-facilitated allergen presentation to T cells, strong activating allergen-specific T cells, and secreting Th2-driving cytokines. Therefore, further research is needed to determine whether increased CD23+ levels increase the risk of developing allergies in stunted children.
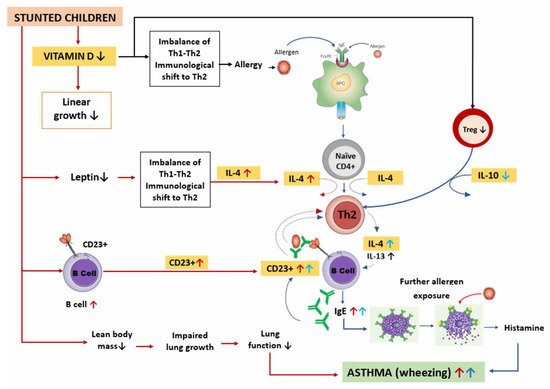
Therefore, the possible mechanisms of asthma in stunted children described above can be summarized as in Figure 4.
Figure 4. Possible mechanism of asthma in stunted children. Stunted children have lower vitamin D levels, resulting in decreased linear growth. Low vitamin D levels cause changes in the Th1–Th2 balance toward Th2, promoting allergic diseases including asthma. The pathophysiology of asthma begins with exposure to allergens captured by APC, which are then presented to naïve CD4+ cells. With the help of IL-4, naïve CD4+ cells differentiate into Th2 cells that produce IL-4 and IL-13. Thereafter, B lymphocytes differentiate into plasma cells that produce IgE and attach to the mast cells. Further exposure to allergens and crosslinking of allergens with IgE occur on the outer surface of membrane of mast cells, resulting in degranulation, histamine release, and bronchoconstriction, leading to asthma symptoms such as wheezing. In addition, when vitamin D levels are low, the number of Tregs decreases, thereby lowering IL-10 production. This causes an increase in Th2 activity and IL-4, resulting in an increase in IgE, which further stabilizes the CD23+ B cells. B cells take up allergen–IgE complexes and present the allergen to Th2 cells, thereby increasing the Th2 response. In stunted children, there is a decrease in lean body mass that causes impaired lung growth and decreased lung function. In addition, low leptin levels cause changes in the Th1/Th2 balance toward Th2 cells, thereby increasing IL-4 production. B cells also increase, causing an increase in CD23+ levels. All these factors contribute to asthma occurrence in stunted children.
This entry is adapted from the peer-reviewed paper 10.3390/medicina58091236