Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Mechanical
Hot corrosion is due to the severe deterioration of metals due to sulfidation or oxidation reactions of the deposits in the form of liquid or semi-liquid at an operating temperature. In a gas turbine, there are corrosive particles in the environment and lower-grade fuel—for example, chlorine, sodium, sulfur, and vanadium.
- thermal spray coating
- high-velocity oxyfuel
- hot corrosion sulfidation
1. Introduction
Thermal spray is a coating process by which the fine particles of a material in metal or non-metal form are deposited on the surface of a formulated substrate in a molten or semi-molten state [1]. The material to be coated can be deposited in various states, particularly wire, powders, rods, suspension, or molten form [2]. The torch converts particles depending on the energy supplied into a hot gas stream. For combustion, chemical energy is used, and electric power is used for plasma generation [3]. The coating material heated during combustion or plasma generation converts to a molten state and is propelled by the high velocity and high-temperature gas stream to the substrate. The particles distort when depositing the substrate’s surface to produce a splat. When new particles reach the surface, they combine to make multiple splat layers to complete the coating [4]. Figure 1 shows the thermal spray coating process. While selecting a coating material, it should be kept in mind that the thermal expansion coefficient of the substrate and the coating material must match each other [5].
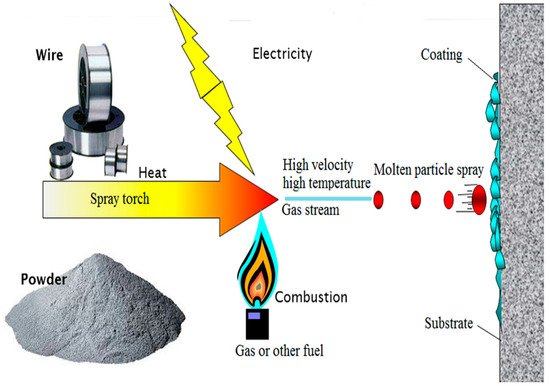
Figure 1. Thermal spray process. Modified with permission from [6]. 2007, Sudhangshu Bose.
Naturally, thermal barrier coating contains four layers: a ceramic topcoat, a transitional metallic bond coat, a thermally grown oxide (TGO) layer, and a high-temperature base structural material made of superalloy [7]. The topcoat ceramic performs the function of an insulator, keeps the underlying substrate away from degradation due to the high temperature by providing protection, and resists heat transfer [8,9,10,11,12]. The bond coat is used for oxidation resistance [13,14]. The excessively frequently used materials for the topcoat and bond coat are the materials that are heat resistant, such as yttria-stabilized zirconia (8YSZ) and MCrAlY (M: cobalt, nickel, or a combination of both elements), respectively [8]. Thermal spray techniques deposit the topcoat and bond coat on the metallic substrate [15]. The thermally grown oxide (TGO) layer is a barrier against oxygen diffusion [16,17]. A substrate is usually made of cobalt or nickel superalloy to tolerate the load mechanically [18]. During service, it must be kept in mind that the composition and microstructure of the layers change instantly, leading to the failure and degradation of the coating [19].
The life and performance of service materials have improved with the development and research of new thermal coatings [20,21,22,23,24,25,26,27]. Thakare et al. [18] have presented an overview of thermal spray coating materials and technology, the progression in deposition techniques, and the materials used in thermal spray coating. They also introduced the TBC system’s residual stresses, properties, high-temperature performance, and life prediction models. Wu et al. [28] summarized the preparation procedures and improvements in new ceramic materials as well as current problems and future trends. Mehboob et al. [7] reviewed the failure mechanisms of TBCs and methods to alleviate these failures. Gopi et al. [29] addressed the corrosion analysis, wear characteristics, hardness, microstructure, and surface roughness of tungsten carbide coatings sprayed by high-velocity oxyfuel coating. Gupta N. et al. [30] reviewed the mechanical and microstructural properties of the CrC coatings coated by HVOF and their suitability for the applications of piston rings. Vats et al. [31] investigated the outcome of deposition process parameters on the mechanical, wear, physical, corrosion, and erosion properties of coatings deposited by HVOF. Kumar et al. [32] presented an analysis of nanocomposite coatings sprayed by HVOF and concluded that nanocomposite coatings sprayed by HVOF can alleviate the erosion of traditional coatings.
2. Hot Corrosion in Gas Turbine Blades
Due to combustion gases in gas turbines, the environment is not clean [33]. There are impurities in the fuel or combustion air, such as sea water aerosol containing vanadium and sulfates, deposited on the surface of turbine blades. Hot corrosion is due to the severe deterioration of metals due to sulfidation or oxidation reactions of the deposits in the form of liquid or semi-liquid at an operating temperature. Corrosion has a destructive impact on the structure of components [34]. Corrosion can be prevented by using protective measurements [35].
In the case of gas turbines, the temperature can reach 1500 °C. Typically, the material used for coating is made of YSZ, which is stable up to 1200 °C. Above this temperature, the coating starts to damage. Due to this, the turbine’s lifetime is decreased at high temperatures. The corrosion of superalloys or materials in the presence of oxidizing gas due to molten salt at a high temperature of 700–925 °C is called hot corrosion. The oxidation rate is fast at this temperature when alloys and metals are contaminated with salts. As a result, the protective oxide layer breaks down, and salts reach the metal surface, causing degradation [36]. Due to this degradation, the bond coat and topcoat are affected because of the porosity of the APS method in YSZ coating. Through pores and cracks, the molten salt penetrates the YSZ and reacts with a bond coat. [37] It is different from corrosion at low temperatures. Suppose the hot corrosion occurs at a temperature equal to the melting point of salt layers. In that case, it is hot corrosion of type 1, and the corrosion occurring at a temperature less than the melting point of the salt layers is called type 2 hot corrosion. The temperature range for high-temperature corrosion is 800 to 900 °C, and for low temperatures, it is 700 to 800 °C. Figure 2 represents the hot corrosion mechanism at low and high temperatures of a substrate.
In a gas turbine, there are corrosive particles in the environment and lower-grade fuel—for example, chlorine, sodium, sulfur, and vanadium [38]. Corrosion in the topcoat is because of lead (Pb) and vanadium in the fuel. Vanadium forms vanadium pentoxide by reacting with oxygen. Sulfur is also present in the fuel that reacts with NaCl and oxygen in the air intake to produce Na2SO4. These two products are hazardous for the topcoat [39]. These species have pits and voids at the grain boundary responsible for the flow of corrosive particles that react with the metal to deteriorate them, and in this way, the life of the components is decreased. Corrosion in the bond coat can be produced due to chlorides and sulfates of sulfur or potassium gas. In the air, potassium or sodium chloride reacts with sulfur and oxygen in the fuel to have potassium or sodium sulfates. These sulfates are the reason for corrosion in the bond coat. Despite the fuel or air, corrosion can also be produced after combustion due to the contaminants in the fuel that deposit on the surface. After that, these corrosive products stick on the surface of hot sections such as turbine blades. A non-defensive porous oxide scale is produced on the surface, and the material is consumed quickly. As a result, the ability of a material to carry a load is diminished, which is the reason for the failure of the components.
On the other hand, molten deposits are also responsible for the hot corrosion of thermal barrier coatings. The first mode of corrosion by molten deposits is chemical reaction attack by-products—for example, vanadium and sulfur. These products react with ceramic oxides to form corrosive melts. The second mode is mineralization due to the phase reaction of nonreactive liquid, which moves the nonequilibrium phase to the equilibrium phase. The third mode is the corrosion of the bond coat, in which corrodent melts or corrosive gases can enter the zirconia layer under some conditions and corrode the bond coat, eventually leading to the failure of TBC. The fourth mode is the physical damage of coating by the penetration of molten salt through the grains of TBC [40]. So, there is a need to keep the hot segment parts away from molten salt particles at high temperatures. A potentiodynamic polarization test in the saline solution measures the corrosion resistance. The current density for corrosion is between the range of 10−5 to 10−6 A/cm2 [41]. If the turbine blade is not coated, then pitting and corrosion can affect the blade, and the consequences may be severe if there is a salty environment.
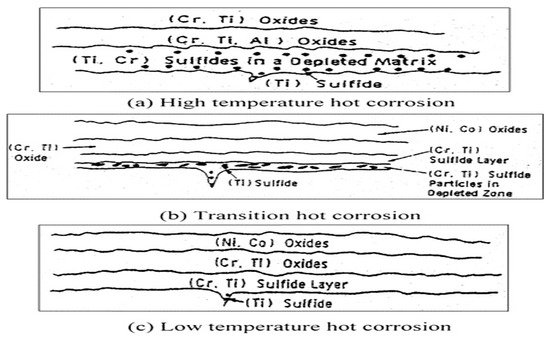

Figure 2. Hot corrosion mechanism in a gas turbine blade. Reprinted with permission from [42]. 2004, M.R Khajavi, M. H Shariat.
There are two stages of hot corrosion in coatings and alloys. These are the initiation stage and the propagation stage. The initiation stage is also called the incubation period. The protective scale is produced at the start of this stage, which variates with time. Each metal may grow its composition and structure of the scale in this period, so it is considered a clean metal surface in an actual gas turbine. Usually, the superalloys that produce protective scales are alumina formers and chromia formers. The length of the initiation stage is affected by many parameters—for example, the fabrication condition, alloy composition, gas velocity and composition, specimen geometry, erosion, temperature cycling and temperature, salt deposition rate, and composition and condition of salt. The protective scale may break down due to some mechanisms. One is basic and acidic fluxing of the scale or a portion of it. Other mechanisms to be considered are the formation of sulfide or growth stress under the scale, thermal cycling due to mechanical cracking of the scale, and modification of the scale due to chloride. The duration of the first stage depends on several factors—for instance, the fuel-air ratio, the content of chlorides in the fuel impurities and the S content of the fuel, the intake air, the level of contaminant of the fuel, the composition, the quantity of deposit, the difference in temperature between the flue gas and the surface of metal, and the composition and temperature of the material.
The same parameters are responsible for the corrosion rate as for the initiation stage for the propagation stage. Corrosion resistance can be improved by increasing the chromium content, which helps extend the incubation period and reduces the corrosion rate in the propagation stage. However, the W and Mo contents can destroy the corrosion resistance property of chromium if they are about 5% in value by supporting the acidic fluxing of alloy. The corrosion rate is affected by heat flux. If the blades are cooled, there is a chance of an increased deposition rate, and a shift into a region with acidic fluxing of low temperatures with high corrosion rates should be avoided. Internal sulfidation due to the transport of sulfur crossways to the protective scale is the reason for the cracking of the scale because of produced stresses in the scale [43].
This entry is adapted from the peer-reviewed paper 10.3390/ma15186329
This entry is offline, you can click here to edit this entry!
