Gastric cancer is a common type of cancer that poses a serious threat to human health. Polysaccharides are important functional phytochemicals, and research shows that polysaccharides have good anti-gastric cancer effects. Researchers collated all relevant literature published from 2000 to 2020 and found that more than 60 natural polysaccharides demonstrate anti-gastric cancer activity. At the present, the sources of these polysaccharides include fungi, algae, tea, Astragalus membranaceus, Caulis Dendrobii, and other foods and Chinese herbal medicines. By regulating various signaling pathways, including the PI3K/AKT, MAPK, Fas/FasL, Wnt/β-catenin, IGF-IR, and TGF-β signaling pathways, polysaccharides induce gastric cancer cell apoptosis, cause cell cycle arrest, and inhibit migration and invasion. In addition, polysaccharides can enhance the immune system and killing activity of immune cells in gastric cancer patients and rats.
- anti-gastric cancer
- polysaccharides
- plants
- fungi
1. Introduction
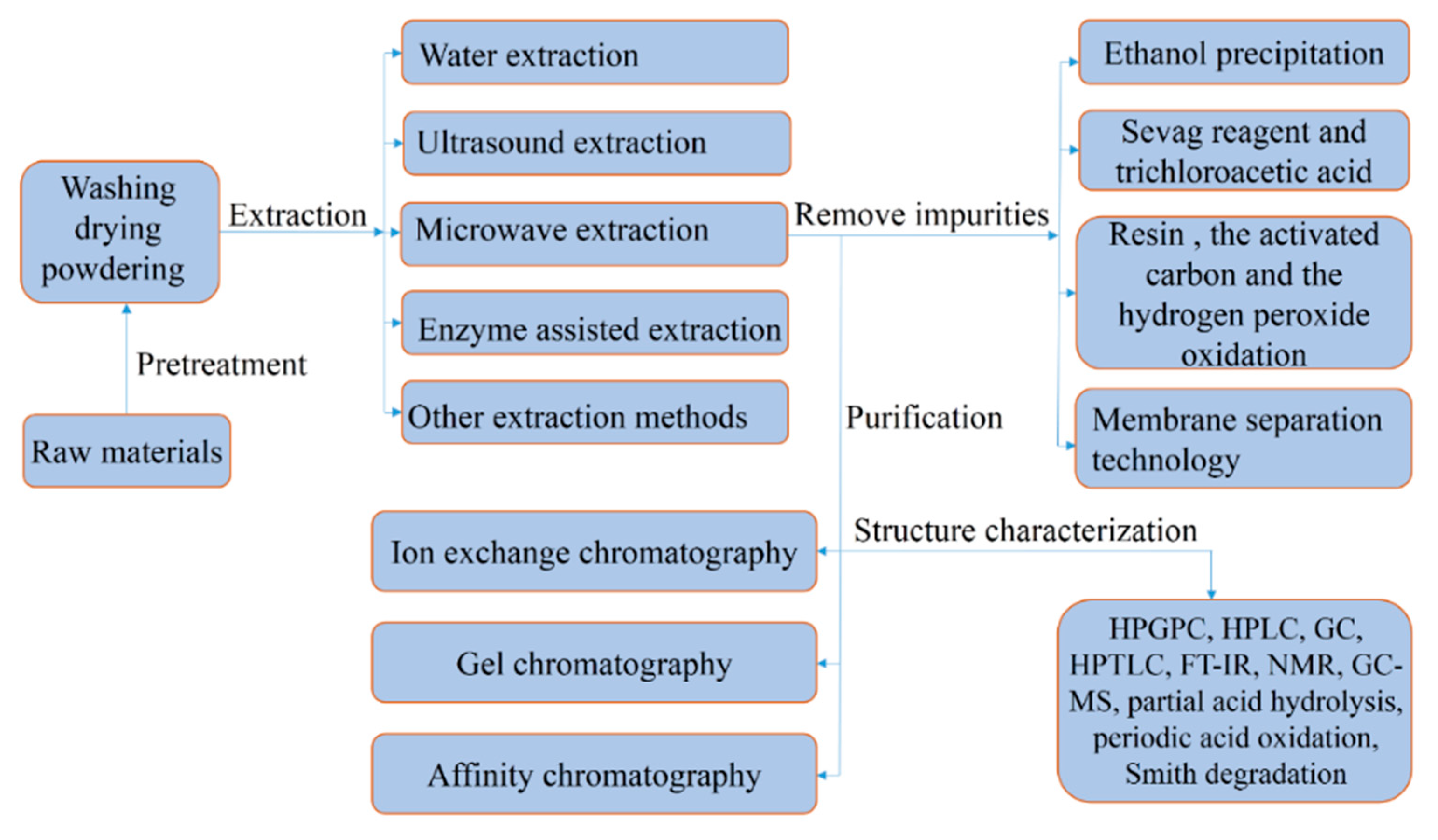
2. Extraction of Polysaccharides
2.1. Ultrasonic Extraction Method
2.2. Microwave Extraction Method
2.3. Enzyme-Assisted Extraction
2.4. Other Extraction Methods
3. Purification of Polysaccharides
4. Structural Characterization of Polysaccharides
This entry is adapted from the peer-reviewed paper 10.3390/molecules27185828
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide For 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Han, S.Y.; Youker, S. Metallic Taste as a Side Effect of Topical Fluorouracil Use. J. Drugs Dermatol. 2011, 10, 1201–1203.
- Rösch, S.; Werner, C.; Müller, F.; Walter, P. Photoreceptor Degeneration by Intravitreal Injection of N-methyl-N-nitrosourea (MNU) in rabbits: A pilot Study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 255, 317–331.
- Gallardo, A.C.; Rodríguez, R.M.; Pacheco, C.C.; Satue, C.G.; Servio, L.I. Dermatological Side Effects of Intravesical Mitomycin C: Delayed Hypersensitivity. Arch. Espanoles Urol. 2016, 69, 89–91.
- Hong, W.; Kim, K.; Jung, Y.; Kim, J.; Kang, S.; Chun, J.; Chun, M.; Yim, H.; Kang, D.; Kim, T. 432 Comparison of Efficiency and Side Effect of Adriamycin and Doxetaxel and Adriamycin, Cyclophosphamide and Paclitaxel in Patients with Locally Advanced Breast Cancer Receiving Neoadjuvant Chemotherapy. Eur. J. Cancer 2012, 48, S172–S173.
- Doval, D.; Sharma, S.K.; Kumar, M.; Khandelwal, V.; Choudhary, D. Cytarabine Ears—A Side Effect of Cytarabine Therapy. J. Oncol. Pharm. Pract. 2019, 26, 471–473.
- Schepetkin, I.A.; Quinn, M.T. Botanical polysaccharides: Macrophage Immunomodulation and Therapeutic Potential. Int. Immunopharmacol. 2006, 6, 317–333.
- Huh, S.; Lee, S.; Choi, S.J.; Wu, Z.; Cho, J.-H.; Kim, L.; Shin, Y.S.; Kang, B.W.; Kim, J.G.; Liu, K.; et al. Quercetin Synergistically Inhibit EBV-Associated Gastric Carcinoma with Ganoderma lucidum Extracts. Molecules 2019, 24, 3834.
- Nie, X.; Shi, B.; Ding, Y.; Tao, W. Antitumor and Immunomodulatory Effects of Weikangfu Granule Compound in Tumor-Bearing Mice. Curr. Ther. Res. 2006, 67, 138–150.
- Yue, H.; Liu, Y.; Qu, H.; Ding, K. Structure Analysis of a Novel Heteroxylan from the Stem of Dendrobium Officinale and Anti-Angiogenesis Activities of Its Sulfated Derivative. Int. J. Biol. Macromol. 2017, 103, 533–542.
- Zhang, Z.; Wang, F.; Wang, X.; Liu, X.; Hou, Y.; Zhang, Q. Extraction of the Polysaccharides from Five Algae and Their Potential Antioxidant Activity In Vitro. Carbohydr. Polym. 2010, 82, 118–121.
- Yang, W.; Pei, F.; Shi, Y.; Zhao, L.; Fang, Y.; Hu, Q. Purification, Characterization and Anti-Proliferation Activity of Polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2012, 88, 474–480.
- Wang, J.; Jia, J.; Song, L.; Gong, X.; Xu, J.; Yang, M.; Li, M. Extraction, Structure, and Pharmacological Activities of Astragalus Polysaccharides. Appl. Sci. 2018, 9, 122.
- Rahimi, F.; Tabarsa, M.; Rezaei, M. Ulvan from Green Algaeulva Intestinalis: Optimization of Ultrasound-Assisted Extraction and Antioxidant Activity. J. Appl. Phycol. 2016, 28, 2979–2990.
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules 2019, 24, 3122.
- Amanda, D.S.E.S.; Weuller, T.D.M.; Laís, M.M.a.; Maria, V.P.R.; Ana, K.P.B. Microwave-Assisted Extraction of Polysaccharides from Arthrospira (Spirulina) Platensis Using the Concept of Green Chemistry. Algal Res. 2018, 35, 178–184.
- Tsubaki, S.; Oono, K.; Hiraoka, M.; Onda, A.; Mitani, T. Microwave-Assisted Hydrothermal Extraction of Sulfated Polysaccharides from Ulva spp. And Monostroma Latissimum. Food Chem. 2016, 210, 311–316.
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-Assisted Extraction of Sulfated Polysaccharides (Fucoidan) from Brown Seaweed. Carbohydr. Polym. 2011, 86, 1137–1144.
- Xu, S.-Y.; Huang, X.; Cheong, K.-L. Recent Advances in Marine Algae Polysaccharides: Isolation, Structure, and Activities. Mar. Drugs 2017, 15, 388.
- Baik, J.H.; Shin, K.-S.; Park, Y.; Yu, K.-W.; Suh, H.J.; Choi, H.-S. Biotransformation of Catechin and Extraction of Active Polysaccharide from Green Tea Leaves Via Simultaneous Treatment with Tannase and Pectinase. J. Sci. Food Agric. 2014, 95, 2337–2344.
- Chen, J.; Li, J.; Sun, A.-D.; Zhang, B.-L.; Qin, S.-G.; Zhang, Y.-Q. Supercritical CO2 Extraction and Pre-Column Derivatization of Polysaccharides from Artemisia Sphaerocephala Krasch. Seeds Via Gas Chromatography. Ind. Crop. Prod. 2014, 60, 138–143.
- Zhao, T.; Luo, Y.; Zhang, X.; Zhang, W.; Qu, H.; Mao, G.; Zou, Y.; Wang, W.; Li, Q.; Chen, Y.; et al. Subcritical Water Extraction of Bioactive Compounds from Radix Puerariae and Optimization Study Using Response Surface Methodology. Chem. Eng. Commun. 2019, 206, 1218–1227.
- Martins, M.; Vieira, F.A.; Correia, I.; Ferreira, R.A.S.; Abreu, H.; Coutinho, J.A.P.; Ventura, S.P.M. Recovery of Phycobiliproteins from the Red Macroalga Gracilaria sp. Using Ionic Liquid Aqueous Solutions. Green Chem. 2016, 18, 4287–4296.
- Zhang, L.; Tu, Z.-C.; Wang, H.; Kou, Y.; Wen, Q.-H.; Fu, Z.-F.; Chang, H.-X. Response Surface Optimization and Physicochemical Properties of Polysaccharides from Nelumbo Nucifera Leaves. Int. J. Biol. Macromol. 2015, 74, 103–110.
- Chen, X.; Nie, W.; Yu, G.; Li, Y.; Hu, Y.; Lu, J.; Jin, L. Antitumor and Immunomodulatory Activity of Polysaccharides from Sargassum fusiforme. Food Chem. Toxicol. 2012, 50, 695–700.
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and Fractionation of Exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350.
- Chen, Z.-G.; Zhang, D.-N.; Zhu, Q.; Yang, Q.-H.; Han, Y.-B. Purification, Preliminary Characterization And In Vitro Immunomodulatory Activity of Tiger Lily Polysaccharide. Carbohydr. Polym. 2014, 106, 217–222.
- Usoltseva, R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The Comparison of Structure and Anticancer Activity In Vitro Of Polysaccharides from Brown Algae Alaria marginata and A. Angusta. Carbohydr. Polym. 2016, 153, 258–265.
- Di, T.; Chen, G.; Sun, Y.; Ou, S.; Zeng, X.; Ye, H. Antioxidant and Immunostimulating Activities In Vitro of Sulfated Polysaccharides Isolated from Gracilaria Rubra. J. Funct. Foods 2017, 28, 64–75.
- Hahn, T.; Zayed, A.; Kovacheva, M.; Stadtmüller, R.; Lang, S.; Muffler, K.; Ulber, R. Dye Affinity Chromatography for Fast and Simple Purification of Fucoidan from Marine Brown Algae. Eng. Life Sci. 2015, 16, 78–87.
- He, X.; Fang, J.; Ruan, Y.; Wang, X.; Sun, Y.; Wu, N.; Zhao, Z.; Chang, Y.; Ning, N.; Guo, H.; et al. Structures, Bioactivities and Future Prospective of Polysaccharides from Morus Alba (White Mulberry): A Review. Food Chem. 2018, 245, 899–910.
- Liu, Y.; Hu, C.-F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.-T.; Huang, W. Isolation, Characterization and Antioxidant of Polysaccharides from Stropharia Rugosoannulata. Int. J. Biol. Macromol. 2019, 155, 883–889.
- Yin, J.; Lin, H.; Li, J.; Wang, Y.; Cui, S.W.; Nie, S.; Xie, M. Structural Characterization of a Highly Branched Polysaccharide from the Seeds of Plantago Asiatica L. Carbohydr. Polym. 2012, 87, 2416–2424.
- Hu, J.-L.; Nie, S.-P.; Wu, Q.-M.; Li, C.; Fu, Z.-H.; Gong, J.; Cui, S.W.; Xie, M.-Y. Polysaccharide from Seeds of Plantago asiatica L. Affects Lipid Metabolism and Colon Microbiota of Mouse. J. Agric. Food Chem. 2013, 62, 229–234.
