Bray et al. evaluated global cancer incidence and mortality according to GLOBOCAN 2018, provided by the International Agency for Research on Cancer. Research showed that gastric cancer is still a serious threat to human health. There are more than one million new cases of gastric cancer and more than 700,000 deaths reported worldwide every year. Gastric cancer has become the fifth most frequently diagnosed cancer, and it is the third leading cause of cancer-related death [
1]. At present, the clinical treatment of gastric cancer is largely chemotherapy, but these medicines cause adverse reactions. Fluorouracil can cause a loss of appetite, nausea, vomiting, and diarrhea [
2]. Nitrosourea can damage liver and kidney functions [
3]. Mitomycin and adriamycin are cardiotoxic [
4,
5]. Cytarabine can cause myelosuppression and adverse gastrointestinal reactions [
6]. Therefore, researchers are looking towards natural medicines as potential medicines with higher anti-gastric cancer activity and lower toxicity. Currently, polysaccharides are used in biochemistry and medicine due to their remarkable therapeutic effects and low toxicity [
7]. Moreover, due to these polysaccharides’ significant anti-gastric cancer activity, they have been used as food additives or medicines [
8,
9].
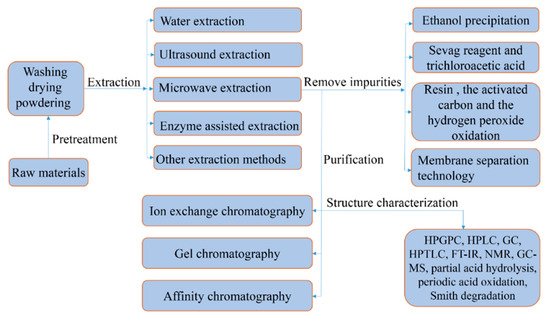
2. Extraction of Polysaccharides
Polysaccharides are polar macromolecules, which are usually soluble in water but not soluble in organic solvents. Therefore, water is currently used as the extraction medium. As they need to be extracted multiple times using high-temperature distilled water, the water extraction method is simple and easy to operate. In addition, research shows that the use of acid or alkaline solutions instead of distilled water can improve the extraction rate of polysaccharides under certain circumstances [
10]. However, although the water extraction method is suitable for almost all polysaccharides, the extraction time can be as long as 2–4 h, which causes the method to take a long time [
11]. According to the basic principle of polysaccharide extraction, that is, destroying the cell wall and causing the polysaccharides to enter the solvent, many new extraction techniques have begun to appear.
2.1. Ultrasonic Extraction Method
This method uses cavitation to destroy the cell wall to accelerate the dissolution of polysaccharides. Using this method can increase the yield of polysaccharides and decrease the extraction time [
12]. By summarizing the research progress on the ultrasonic extraction of Astragalus polysaccharides, Wang et al. found that ultrasonic power had the highest impact on ultrasonic extraction, followed by extraction temperature and extraction time [
13]. Therefore, many researchers will screen for the best extraction conditions when using ultrasonic extraction methods [
14]. However, it should be noted that exposure to an ultra-sonic environment for a long duration changes the structure of polysaccharides and affects their biological activity [
15].
2.2. Microwave Extraction Method
When the energy carried by microwaves continues to act on cells, it can increase the intracellular pressure break the cells within a short period of time, and active ingredients such as polysaccharides can flow into the solvent [
16]. However, rapid temperature change is very likely to change the molecular weight distribution and the structure of thermally unstable polysaccharides. Research on the microwave extraction of seaweed polysaccharides confirms that this method degrades polysaccharides, resulting in changes in their molecular weight and viscosity [
17]. However, microwave extraction is also useful. By changing the microwave power, extraction time, and other factors, researchers can control the degradation rate, sulfate content, viscosity, and molecular weight of seaweed polysaccharides within the required range to obtain the required seaweed polysaccharides [
18].
2.3. Enzyme-Assisted Extraction
This method destroys the cell wall and intracellular structure through enzymatic hydrolysis to obtain more polysaccharides. The main enzymes that are currently used include Viscozyme, Cellucast, Termamyl, Ultraflo, carragenanase, agarase, amyloglucosidase, xylanase, Kojizyme, Protamex, Neutrase, Flavourzyme, and Alcalase [
19,
20]. At present, this method is often used in combination with other methods, such as microwave extraction and ultrasonic extraction. Since enzymes are selective to the environment, ensuring enzyme activity is one of the key points to consider when using different enzymes together.
2.4. Other Extraction Methods
In addition to the extraction methods mentioned above, there are many new extraction methods that can be applied to polysaccharide extraction, including supercritical CO2 extraction [
21], subcritical water extraction [
22], ionic liquids extraction [
23], and dynamic high-pressure micro-jet technology [
24]. In general, there are no absolute advantages and disadvantages between different extraction technologies. Choosing a suitable extraction technology is not only related to the characteristics of the polysaccharides, but is also inseparable from the extraction conditions controlled by the researchers.
3. Purification of Polysaccharides
Extracted crude polysaccharides contain impurities such as inorganic salts, proteins, and pigments. Impurities seriously affect the evaluation of the relationship between the structure and biological activity of polysaccharides; as such, they need to be removed. In most cases, ethanol precipitation is the first step in polysaccharide purification, as it can remove low-molecular-weight impurities from polysaccharides [
25]. In addition, membrane separation technologies, such as diafiltration and ultrafiltration, are also widely used to remove impurities [
26]. Conventional methods of removing protein use Sevag reagent or trichloroacetic acid to denature and precipitate the protein [
27]. Methods to remove pigments include the resin method, the activated carbon method, and the hydrogen peroxide oxidation method [
15].
To explore the relationship between structure and biological activity, the polysaccharides obtained after removing impurities require further deep purification. At present, the most commonly used method is chromatographic separation. Ion-exchange chromatography is suitable for separating neutral or acidic polysaccharides via gradient salt elution or pH adjustment [
28]. For most anti-gastric cancer polysaccharides, dicthylaminoethyl-cellulose anion exchange column chromatography is used for deep purification. Size exclusion chromatography (also known as gel chromatography) is based on the principle of different molecular weights or molecular size for separation [
29]. Affinity chromatography uses the adsorption difference between different substances and stationary phases for separation [
30].
4. Structural Characterization of Polysaccharides
The physical, chemical, and biological properties of polysaccharides mainly depend on the type, ratio, and sequence of monosaccharides; their molecular weight; the configuration of the glycosidic bonds; the types of glycosidic bonds; and the positions of the glycosidic bonds [
31]. High-performance gel permeation chromatography can not only be used to determine the homogeneity of polysaccharides, but can also be used to determine the molecular weight [
32]. Partial acid hydrolysis, periodic acid oxidation, Smith degradation, high-performance liquid chromatography, gas chromatography, and high-performance thin-layer chromatography are also used to determine the composition of monosaccharides [
33]. Nuclear magnetic resonance spectroscopy is used to determine the ratio of monosaccharides and anomeric bonds [
34]. Gas chromatography-mass spectrometry is used to determine linkage positions [
33]. We summarize the extraction, purification, and characterization steps in
Figure 1.
Figure 1. Schematic representation of the extraction, purification, and characterization of polysaccharides against gastric cancer.