Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Essential oils (EOs) have been widely exploited for their biological properties (mainly as antimicrobials) in the food industry. Encapsulation of EOs has opened the way to the utilization of EOs in the pharmaceutical and biomedical fields. Electrospinning (ES) has proved a convenient and versatile method for the encapsulation of EOs into multifunctional nanofibers.
- electrospinning
- emulsion
- coaxial
1. Introduction
Essential oils (EOs) are complex mixtures of volatile compounds synthesized by plants for defense and signaling purposes [1][2]. EOs exhibit also a broad spectrum of biological properties such as antimicrobial, analgesic, anti-inflammatory, antidiabetic, antitumor at the same time, etc. [3][4][5]. The increasing problem of bacterial resistance to antibiotics has led researchers to the quest for new antimicrobial agents. EOs are characterized in general by accepted safety and efficacy profiles, and therefore they may be promising candidates against microbial infections [1][6][7]. EOs are widely studied for their pharmaceutical applications and recent studies have dealt with their formulation into pharmaceutical solid dosage forms such as tablets [8] with targeted delivery to specific sites of the human body [9]. In addition, EOs hold promise as antimicrobial agents for topical wound healing applications [10]. However, the use of EOs in the pharmaceutical and biomedical fields is still limited due to several drawbacks. EOs are volatile, have strong aromas and a bitter taste, are easily oxidized and in general, they are chemically unstable. These drawbacks are still understudied and hence formulation challenges remain [11][12][13].
Encapsulation via spray drying, coacervation, ionic gelation or electrospinning (ES) in micron-, submicron- or nano- scale systems is a widely applied strategy in food, polymer and pharmaceutical industries pertinent to EO formulation [14][15][16][17]. Encapsulation of EOs can improve their physicochemical stability and organoleptic characteristics and provide controlled/targeted release profiles [7][8][9][18]. Moreover, encapsulation in nanoscale systems could improve bioavailability as a result of improved dissolution in biological liquids and permeation through biological barriers, thus enhancing their biological properties [19][20]. Over the various encapsulation techniques available, ES provides important advantages: (i) it can produce very thin fibers (of few nanometers in diameter) with large specific surface areas (high surface-to-volume ratio) and large interstitial spaces (voids); (ii) it can provide ease of functionalization; (iii) the produced electrospun nanofibers exhibit superior mechanical properties; (iv) it is a versatile process and (v) it is amenable for scale-up [21].
The study of electrospun nanofibers loaded with essential oils (EONFs) arose in the early 2010s aiming mainly at nutraceutical products (e.g., active food packaging) [22]. However, in the last five years (2017–2022) the fabrication of EONFs has gained great interest and attracted many researchers to work in this field. Encapsulation of EOs into polymeric nano/microfibers has enabled new approaches to wound dressings, scaffolds for tissue engineering and controlled/targeted delivery thereof [23]. Controlled release of EOs from nanofibers can also minimize the cytotoxic effects of some EO components on human cells [22][23]. Moreover, EOs can attain stability in the EONF systems and thus render prolongation of product’s shelf-life, thus opening the way to commercialization.
2. Electrospinning of Essential Oils
Electrospinning (ES) technology has been extensively explored and documented in the literature for the fabrication of multifunctional drug delivery systems [24][25]. Consequently, its potential use in the preparation of EONFs is justifiably explored. In the literature, two main ES technologies have been reported for the fabrication of EONFs: (i) the traditional ES set-up and (ii) its coaxial modification. For fabricating EONFs, both natural and synthetic polymers have been used as carrier materials. A wide range of actives (such as small molecular weight active pharmaceutical ingredients—APIs, biological materials, cells or bacteria, etc.) can be electrospun using liquids (such as melts), solutions or suspensions as solvent systems [21][26]. For the fabrication of EONFs, either spinning solutions or emulsions have been utilized. In this section, a brief overview of the set-up and working principle of ES, and the main carrier polymers used for preparing EONFs intended for pharmaceutical and biomedical applications, will be presented and discussed.
2.1. Overview of Electrospinning Set-Ups Utilized for the Fabrication of EONFs
2.1.1. Conventional Electrospinning (ES)
A conventional ES experimental set-up consists typically of four main parts: a high-voltage power supply; a spinneret (usually a metallic needle) fitted to a syringe; a syringe pump, and a grounded collector (usually a copper or aluminum plate) [25][27]. A schematic representation is presented in Figure 1. The high-voltage power supply (typically in the range of 5 to 25 kV [21]) is attached to a spinneret and collector, usually charging positively or negatively the spinneret and oppositely charging the collector. The collector is preferably grounded to enhance the deposition of the newly fabricated nanofibers on its surface [25]. The syringe receives feed liquid (usually a solution) at a constant flow rate controlled by a syringe pump, which subsequently forms a pendant droplet at the tip of the spinneret.
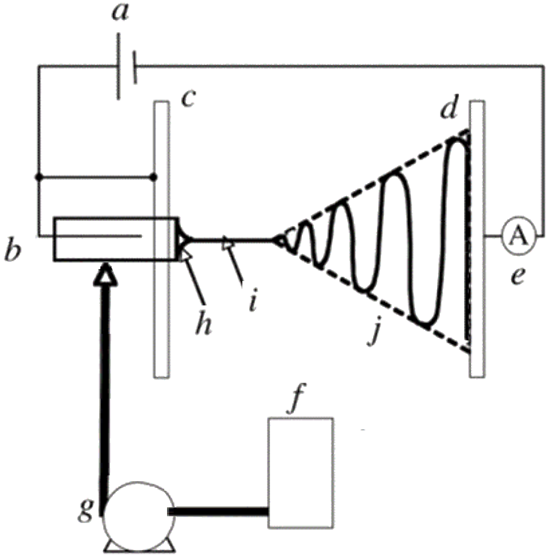
Figure 1. Schematic representation of a conventional ES set-up: (a) high-voltage supply, (b) charging device of the spinneret, (c) high-potential electrode, (d) grounded collector, (e) current measurement device, (f) working solution reservoir, (g) flow rate control of the syringe pump, (h) Taylor’s cone, (i) thinning jet and (j) instability region. Reprinted with permission from [27].
The high voltage applied to the spinneret causes the accumulation of electric charges on the droplet surface, where at some point a critical voltage is reached. When this critical voltage value is reached, the generated electrostatic repulsion overcomes surface tension and viscoelastic forces, and the formation of a cone, known as the Taylor cone, is observed. Therefore, the high-voltage electrical field is essential for the ES process, otherwise, the feed liquid will be extruded from the syringe in the form of spherical droplets [28].
At the apex of the Taylor cone (Figure 1h), the feed solution emits an electrified jet which elongates (a process known as jet thinning; Figure 1i) and is accelerated towards the grounded collector plate, while at the same time the solvent evaporates (Figure 1j) inducing jet solidification. At the end of the process, solid nano/microfibers are deposited on the collector surface forming usually a non-woven fibrous mat [25][27][28].
The parameters affecting the ES process have been widely explored in the literature and discussed in depth in a number of review articles (e.g., [28][29][30][31]). These include the properties of spinning liquid (viscosity, molecular weight of the polymer, conductivity and volatility of the solvent system), the applied voltage, the pumping rate, the spinneret to collector distance, the environmental temperature and humidity, and the collector design and geometry [21]. Lack of control or non-optimization of the aforementioned variables can lead to the formation of beaded—or with other defects—nanofibers [28][32].
2.1.2. Coaxial Electrospinning (CES)
Coaxial electrospinning (CES) is a modification of conventional ES entailing an arrangement of two or more feeding systems to synchronously spin multiple polymeric liquids from coaxial syringe capillaries used as spinneret [33][34]. CES can produce continuous coated or hollow nanofibers suitable for the encapsulation of EOs [35][36]. Since CES is a modification of the conventional ES technology, they are conceptually similar. They share a similar main set-up, where the quality and morphology of the electrospun nanofibers are governed by the same parameters [34][37][38]. However, in the case of CES, the spinneret shows a more sophisticated design and geometry to enable the simultaneous spin of multiple feed liquids.
The first CES set-up was introduced and developed by Sun et al. (2003) [35]. In this set-up, a core-shell needle was utilized as the spinneret, which was attached to a double-compartment syringe. The experimental set-up of the first developed CES device is presented in Figure 2. As seen in Figure 2, the main geometry of a CES spinneret is designed by the insertion of a needle or capillary (inner needle) into a concentric outer needle, which communicate with the core and shell feed reservoirs, respectively [34][38].
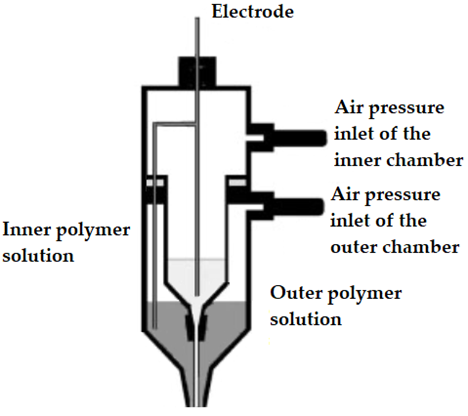
Figure 2. Original experimental set-up used for the first time in a coaxial electrospinning process (CES). Adapted with permission from [35].
As described in the case of conventional ES, the application of a high-voltage electric field will result in a Taylor cone. However, in the case of CES, the formed cone consists of the core liquid surrounded by the wall/shell liquid. A schematic illustration of the formation of this compound Taylor cone is shown in Figure 3a. The real-time photos of jet initiation and thinning from a compound Taylor cone by voltage increase, are presented in Figure 3b. On its way to the collector, the jet experiences bending instability and follows a back-and-forth whipping trajectory, during which the two solvents evaporate, and complex nanofibers are formed [39]. As the compound Taylor cone remains stable, the core is uniformly embedded into the wall/shell, thus resulting in the formation of core-sheath nanofibers.
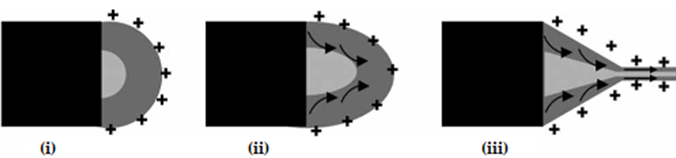
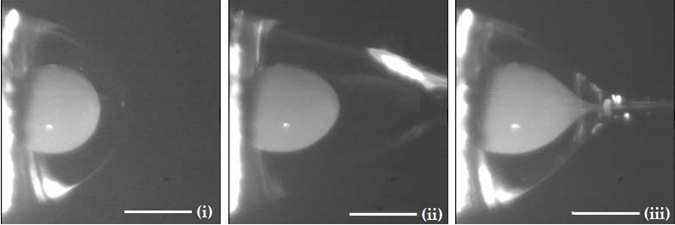
Figure 3. (a) Schematic illustration of the formation of a compound Taylor cone: (i) Surface charges on the sheath solution, (ii) Viscous drag exerted on the core by the deformed sheath droplet, (iii) Sheath-core compound Taylor cone formed due to continuous viscous drag). Adapted with permission from [39]. (b) Formation of compound Taylor cone menisci and the electrified coaxial jet. Voltage increases from (i) to (iii) (scale bar 0.5 mm). Adapted with permission (CC BY 4.0) from [40].
2.1.3. Advantages of ES and CES EONFs over Traditional Polymeric Films
ES and CES technologies for the fabrication of EONFs have shown a number of advantages compared to the traditional polymeric films loaded with EOs [8][21][36][39][41][42][43][44]:
- Improved isolation of the physically and chemically unstable EO and minimization of the chances of decomposition under highly reactive environmental conditions;
- Better sustained/prolonged or targeted release characteristics;
- Reinforcing the elastic and oily nature of EOs to improve their mechanical properties (and thus enhancing e.g., their densification and compression into tablets);
- Ability to serve as scaffolds for biomedical applications in which the less biocompatible EO is efficiently encompassed by a biocompatible polymeric material.
2.2. Spinning Emulsions in the Fabrication of EONFs
The description of the conventional ES and CES until this point was made assuming that the feed liquid was a polymeric solution. However, for the preparation of EONFs, both solution and emulsion ES have been used. As already discussed, in solution ES, the solidification of electrospun material(s) is based on rapid solvent evaporation. This is associated with some limitations related to solvents’ toxicity, environmental concerns and additional solvent extraction processes [45]. Moreover, spinning solutions in ES have been reported to show low production yield compared to melt ES [45][46]. Many strategies have been proposed to deal with the low productivity of solution—based ES, including multi-needle systems and needleless ES systems [21][45]. The approach of emulsion—based electrospun nanofibers was first reported by Sanders et al. (2003) [47] and this approach has recently drawn the attention of some research groups for the preparation of EONFs.
Emulsion-based ES (EES) technology involves the same basic set-up as conventional/solution or coaxial ES. The major difference, however, is that in EES a synchronous spinning of two immiscible solutions is implied. The fiber-forming polymer is dissolved in aqueous solvent(s) to form a continuous phase (O/W emulsions), while the EO is either solubilized in organic non-polar solvent(s) or used as such to form the internal phase. During EES, the continuous phase evaporates rapidly (as in solution ES) resulting in a viscosity increase. Thus, the internal phase droplets will migrate to the center of the jet. The droplets are then merged due to the mutual dielectrophoresis resulting in core-sheath structured nanofibers [48]. EES is more complex compared to ES due to the requirements of special spinnerets. Solvent and surfactant selection in EES is more time-consuming than in ES, since the preparation of physically stable and spinnable emulsions is critical. The type (ionic or non-ionic) and concentration of the solvent(s) and surfactant(s) are expected to affect the surface tension and the conductivity of emulsion, and ultimately the topology and the internal architecture of the EONFs [49].
It is evident that the generation of nano/microfibers by EES leads to the same nanofiber structures as in coaxial ES (CES). However, compared to CES, EES may damage the EO due to the interfacial tension between the two immiscible phases of the emulsion [45][50]. In Figure 4, a schematic illustration of spinnerets loaded with different spinning liquids for (i) conventional (traditional) ES, (ii) CES and (iii) EES is presented to better visualize the differences between the three methods utilized for the preparation of EONFs.
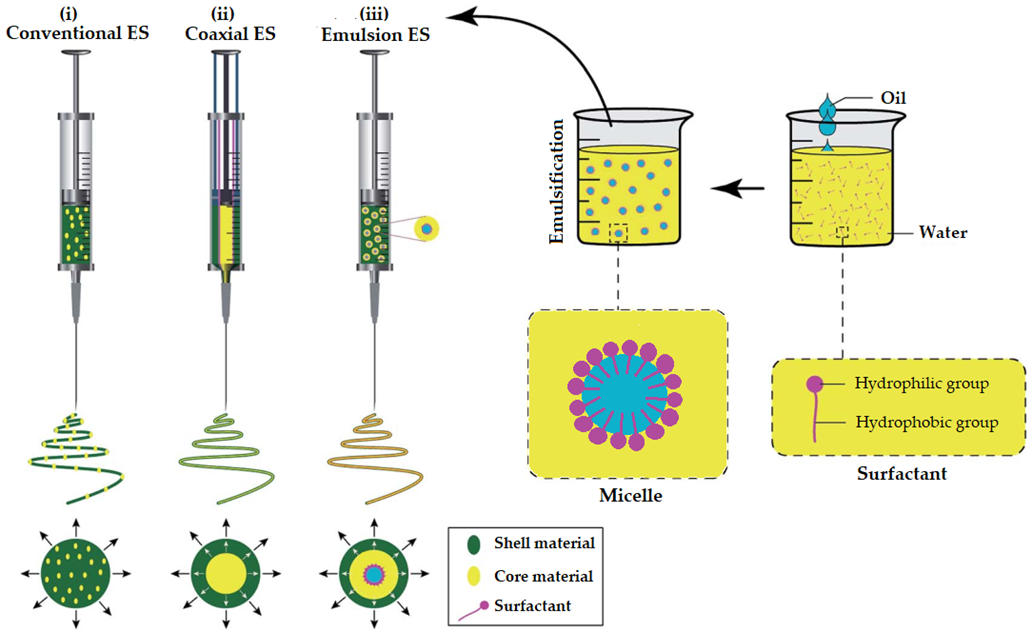
Figure 4. Schematic illustration of spinnerets loaded with different spinning liquids for (i) conventional (traditional), (ii) coaxial and (iii) emulsion electrospinning (ES). Adapted with permission (CC BY 4.0) from [45].
2.3. Biocompatible Polymers Used for the Preparation of EONFs
The studies included and discussed in the following section have utilized a wide range of polymers for the encapsulation of the EOs. Some of the polymers used in these research works are of natural origin, but most of them are synthetic or semi-synthetic. These polymers are also biocompatible, and they have shown high encapsulation efficiency for bioactive compounds. Moreover, their solutions are readily spinnable and for some natural polymers, methods to enhance their solution spinnability are presented. The polymers are presented in alphabetical order in the following sub-sections. Further to the presented polymers here, any other biocompatible, biodegradable and easily spinnable polymers can be used for the preparation of EONFs (e.g., polylactic acid, starch, whey protein isolate, methyl cellulose).
2.3.1. Cellulose Acetate (CA)
Cellulose acetate (CA) is an esterified derivative of cellulose, which offers superior properties for ES compared to other available cellulosic derivatives [51]. Cellulose itself is insoluble in most of the common solvents due to the presence of inter- and intramolecular H-bonds (resulting in gel structures), which makes it challenging to be used in ES. Unfortunately, the solvents in which cellulose is soluble give solutions that are not spinnable [52]. Acetone is an exception, enabling the fabrication of cellulosic nanofibers. Moreover, ionic liquid solutions of CA were able to improve spinnability.
2.3.2. Chitosan (CHS)
Chitosan (CHS) is the deacetylated derivative of chitin, which is the second most abundant polysaccharide after the similarly structured cellulose. CHS is soluble in organic acid solutions as well as in water, ethanol and acetone in the presence of a small amount of acid. CHS solutions are highly viscous, due to their amino groups that are positively charged in the pH range of 2 to 6 [53], which is a limitation for its use in ES [54]. Moreover, the formation of H-bonds in a 3D network restricts its chains’ movements in the electrical field applied during the ES process [55][56]. Thus, the selection of the right grade of CHS with suitable molecular weight and solution viscosity, along with suitable solution concentration is critical and needs to be optimized [54]. Mixing CHS with a variety of synthetic polymers, metal nanoparticles, metal oxides, zeolite or organic metal structures, could also enhance its solution properties and spinnability [57].
2.3.3. β-Cyclodextrin (βCD) Derivatives
Beta-cyclodextrin (βCD) is a cyclic oligomer of glucopyranose structured as a hollow truncated cone. Its interior shows a partially hydrophobic character while its exterior is hydrophilic due to the presence of hydroxyl groups [58][59]. Among the different cyclodextrin grades, βCD is the less water-soluble one [60]. To overcome this limitation and enhance its spinnability, βCD has been chemically modified (e.g., methylated-βCD and 2-hydroxypropyl-βCD) [58][59][61]. An interesting aspect of βCD is that it can generate polymer-free electrospun nanofibers, thus avoiding the necessity to use high molecular weight polymers that will traditionally enable entanglements to ensure the formation of defect-free nanofibers [59].
2.3.4. Gelatin (GEL)
Gelatin (GEL) is the hydrolyzed derivative of collagen and it is a polyampholyte protein [62][63]. Since GEL is derived from collagen, it shows good mechanical properties due to its peptide composition and mimicry [64]. GEL contains arginine-glycine-aspartate (RGD) sequences providing suitable sites for cell attachment [65]. However, GEL shows poor solubility in water forming a gel structure through strong intra- and intermolecular interactions between the polypeptide chains. To overcome this limitation and enable the spinning of its solutions, GEL is usually employed after hydration with hot water using specific ES set-ups, that are able to circulate hot water during the solution feeding process [66][67]. Moreover, to prevent gelation and facilitate electrospinning, GEL can be dissolved in fluorinated alcohols or acidic organic solvents prior to application.
2.3.5. Gellan (GLL)
Gellan (GLL) is a natural exopolysaccharide produced from the bacteria Sphingomonas elodea [68]. GLL shows a complex sol–gel behavior while its aqueous solubilization is characterized as non-typical suggesting difficulties in ES processing [69]. Unstable Taylor cones of GLL solutions in ES have been also reported, and these drawbacks were assigned to the anionic nature, low shear viscosity and strong shear thinning behavior (at low shear rates) of such solutions [70][71].
2.3.6. Polyacrylonitrile (PAN)
Polyacrylonitrile (PAN) also known as polyvinyl cyanide is a synthetic organic polymer resin and the precursor for high-performance carbon fiber, due to its ladder-like structure [72]. PAN has drawn attention due to the high carbon yield and mechanical properties of the produced carbon fibers [73]. It is widely explored in the electrochemical field due to its low electrical conductivity and insulation ability [72][74]. Researchers in this field have utilized PAN as a filament-forming polymer [75][76]. However, its use in the pharmaceutical and biomedical fields remains as an adsorbent of microorganisms, while its use in ES is not common due to its strong static electricity [77].
2.3.7. Poly-ε-caprolactone (PCL)
Polycaprolactone (PCL) is a semi-crystalline linear aliphatic polyester offering combination of polyolefin-like mechanical properties and polyester-like hydrolysability [78]. Its rheological and viscoelastic properties allow its use in the ES process [79]. The hydrophobicity of PCL nanofibers demands modifications to enable higher biocompatibility and hydrophilicity, such as surface coating, plasma treatment, poly(dopamine) treatment, blending with the copolymer, alkali treatment and polymer grafting, making it favorable for a range of biomedical applications [80][81].
2.3.8. Polyethylene Oxide (PEO)
Polyethylene oxide (PEO) is a polyether available in a wide range of molecular weights. MWs < 100,000 are generally called polyethylene glycols (PEGs), whereas higher molecular weight polymers are classified as PEOs [82]. PEOs are amphiphilic and readily dissolve in water and in a variety of organic solvents [83][84]. Due to their high MW, PEOs have been utilized extensively for the encapsulation of small molecules.
2.3.9. Poly(L-lactide-co-ε-caprolactone) (PLCL)
Poly(L-lactide-co-ε-caprolactone) (PLCL) is a copolymer of polylactic acid (PLA) and polycaprolactone (PCL) in a mass ratio of 50:50 and it is usually characterized as a hydrophobic aliphatic polyester copolymer [85][86]. It is often applied as a mechano-stimulating tissue engineering scaffold [87][88][89]. However, its surface lacks adhesive and structural proteins that would enable cell adhesion, proliferation and tissue remodeling [90]. Hence, it has been combined with polymers of natural origins (e.g., collagen) to enhance its biological properties [91].
2.3.10. Polyurethane (PU)
Polyurethanes (PUs) contain the urethane group (–NH–(C=O)–O–) in their structure and are thermosetting polymers [92]. They are capable of strong intermolecular bonding and hence they have been utilized for applications in adhesives and coatings, elastomers, foams and tissue engineering [93]. Electrospun PU nanofibers exhibit good mechanical and adhesion properties and have been used as wound dressing materials in biomedical applications and drug delivery [94].
2.3.11. Polyvinyl Alcohol (PVA)
Polyvinyl alcohol (PVA) is a non-ionic hydrolysis derivative of polyvinyl acetate (PVAc) and it is a highly hydrophilic semicrystalline polymer with excellent mechanical properties [95]. However, PVA itself lacks bioactivity, and in order to produce functional nanofibers, blending with natural polymers or biomolecules is usually required to produce electrospun nanofibers with accelerated wound healing characteristics [96]. PVA shows thermal stability, high viscoelastic properties, good chemical stability and the ability to enhance the mechanical properties of the electrospun nanofibers [97][98].
2.3.12. Polyvinylidene Fluoride (PVDF)
Polyvinylidene fluoride (PVDF) is the polymerization derivative of vinylidene difluoride. PVDF is a highly non-reactive thermoplastic and electroactive polymer [99]. Due to its polar crystalline nature, it is able to produce large voltages with low forces and therefore it is mainly utilized for piezoelectric applications [100][101]. Nevertheless, its applications in the biomedical field are also recognized since it has shown good cell adhesion properties and long-term stability, e.g., for the preparation of multifilament for vascular grafts, ligament and artificial cornea [102] and for smart piezoelectric biomaterials [103][104].
2.3.13. Polyvinyl Pyrrolidone (PVP)
Polyvinyl pyrrolidone (PVP) is the polymerization derivative of N-vinylpyrrolidone. PVP has unique properties of aqueous solubility, as well as in many organic solvents [105][106]. Its water-affinity and good adhesion properties make it one of the most useful materials in the biomedical and pharmaceutical fields [107]. PVP has shown high potential as wound-dressing material since it maintains wound-moisturization preventing dehydration and scab formation [108]. It has also been extensively used as an excipient in a variety of drug-delivery systems [109].
2.3.14. Silk Fibroin (SF)
Silk fibroin (SF) is one of the two types of proteins (the other one is sericin) in the silk, that is produced by various insects including the silkworm. SF forms the filaments of silkworm silk and can be regenerated in various forms, such as gels, powders, fibers or membranes [110]. SF shows several distinctive biological properties, such as good oxygen and water vapor, permeability and minimal inflammatory reaction [111][112]. However, SF shows low hydrophilicity and low mass loss rate, limiting its use in ES [65].
2.3.15. Sodium Alginate (SA)
Sodium alginate (SA) is a natural polymer extracted mainly from brown algae. Chemically it is a linear polysaccharide derivative of alginic acid comprised of 1,4-β-d-mannuronic and α-l-guluronic acids. It shows high hydrophilicity with good aqueous solubility. It has antibacterial properties, and it is non-immunogenic, which explains its wide use in the pharmaceutical field [113][114]. However, ES of SA is a challenging process, due to its poor solubility in organic solvents, high conductivity and surface tension [115].
2.3.16. Zein (ZN)
Zein (ZN) is a group of prolamine proteins found in the endosperm of maize (Zea mays). It shows hydrophobic characteristics (due to the presence of leucine and alanine in its structure), low water vapor permeability and greaseproof properties [116][117]. The ES processing of ZN is challenging due to the frequent clogging of the spinneret when aqueous/ethanol solutions are used and the necessity of using hazardous solvents as alternatives. There are also limitations in reusing its solutions [118].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14091799
References
- 21 CFR 182.20—Essential Oils, Oleoresins (Solvent-Free), and Natural Extractives (Including Distillates). 2012. Available online: https://www.govinfo.gov/content/pkg/CFR-2012-title21-vol3/xml/CFR-2012-title21-vol3-sec182-20.xml (accessed on 10 July 2022).
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160.
- Franklyne, J.S.; Mukherjee, A.; Chandrasekaran, N. Essential oil micro- and nanoemulsions: Promising roles in antimicrobial therapy targeting human pathogens. Lett. Appl. Microbiol. 2016, 63, 322–334.
- Nakatsu, T.; Lupo, A.T.; Chinn, J.W.; Kang, R.K.L. Biological activity of essential oils and their constituents. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 21, pp. 571–631.
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70.
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia coli and Staphylococcus aureus. Evid. Based Complementary Altern. Med. Ecam 2015, 2015, 795435.
- Partheniadis, I.; Karakasidou, P.; Vergkizi, S.; Nikolakakis, I. Spectroscopic examination and release of microencapsulated oregano essential oil. ADMET DMPK 2017, 5, 224–233.
- Partheniadis, I.; Vergkizi, S.; Lazari, D.; Reppas, C.; Nikolakakis, I. Formulation, characterization and antimicrobial activity of tablets of essential oil prepared by compression of spray-dried powder. J. Drug Deliv. Sci. Technol. 2019, 50, 226–236.
- Partheniadis, I.; Zarafidou, E.; Litinas, K.E.; Nikolakakis, I. Enteric Release Essential Oil Prepared by Co-Spray Drying Methacrylate/Polysaccharides—Influence of Starch Type. Pharmaceutics 2020, 12, 12060571.
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine 2018, 38, 57–65.
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177.
- Biddeci, G.; Cavallaro, G.; Di Blasi, F.; Lazzara, G.; Massaro, M.; Milioto, S.; Parisi, F.; Riela, S.; Spinelli, G. Halloysite nanotubes loaded with peppermint essential oil as filler for functional biopolymer film. Carbohydr. Polym. 2016, 152, 548–557.
- Mukurumbira, A.R.; Shellie, R.A.; Keast, R.; Palombo, E.A.; Jadhav, S.R. Encapsulation of essential oils and their application in antimicrobial active packaging. Food Control 2022, 136, 108883.
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243.
- Mishra, M. Handbook of Encapsulation and Controlled Release; CRC Press: Boca Raton, FL, USA, 2015.
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815.
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M.; Potdar, S.B. 1—Current overview of encapsulation. In Encapsulation of Active Molecules and Their Delivery System; Sonawane, S.H., Bhanvase, B.A., Sivakumar, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–8.
- Veiga, R.D.S.D.; Aparecida Da Silva-Buzanello, R.; Corso, M.P.; Canan, C. Essential oils microencapsulated obtained by spray drying: A review. J. Essent. Oil Res. 2019, 31, 457–473.
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381.
- Singh, S. Nanomedicine-nanoscale drugs and delivery systems. J. Nanosci. Nanotechnol. 2010, 10, 7906–7918.
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673.
- Mele, E. Electrospinning of Essential Oils. Polymers 2020, 12, 908.
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of Polymeric Nanofibers Loaded with Essential Oils for Biomedical and Food-Packaging Applications. Int. J. Mol. Sci. 2021, 22, 4017.
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Controll. Rel. 2021, 334, 463–484.
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415.
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57.
- Rutledge, G.C.; Fridrikh, S.V. Formation of fibers by electrospinning. Adv. Drug Deliv. Rev. 2007, 59, 1384–1391.
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C. Nanofibres in Drug Delivery; UCL Press: London, UK, 2018.
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347.
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188.
- SalehHudin, H.S.; Mohamad, E.N.; Mahadi, W.N.L.; Muhammad Afifi, A. Multiple-jet electrospinning methods for nanofiber processing: A review. Mater. Manuf. Processes 2018, 33, 479–498.
- Thompson, C.J.; Chase, G.G.; Yarin, A.L.; Reneker, D.H. Effects of parameters on nanofiber diameter determined from electrospinning model. Polymer 2007, 48, 6913–6922.
- Díaz, J.E.; Barrero, A.; Márquez, M.; Loscertales, I.G. Controlled Encapsulation of Hydrophobic Liquids in Hydrophilic Polymer Nanofibers by Co-electrospinning. Adv. Funct. Mater. 2006, 16, 2110–2116.
- Qin, X. 3—Coaxial electrospinning of nanofibers. In Electrospun Nanofibers; Afshari, M., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 41–71.
- Sun, Z.; Zussman, E.; Yarin, A.L.; Wendorff, J.H.; Greiner, A. Compound Core–Shell Polymer Nanofibers by Co-Electrospinning. Adv. Mater. 2003, 15, 1929–1932.
- Zhang, Y.; Huang, Z.-M.; Xu, X.; Lim, C.T.; Ramakrishna, S. Preparation of Core−Shell Structured PCL-r-Gelatin Bi-Component Nanofibers by Coaxial Electrospinning. Chem. Mater. 2004, 16, 3406–3409.
- Qu, H.; Wei, S.; Guo, Z. Coaxial electrospun nanostructures and their applications. J. Mater. Chem. A 2013, 1, 11513–11528.
- Yarin, A.L. Coaxial electrospinning and emulsion electrospinning of core–shell fibers. Polym. Adv. Technol. 2011, 22, 310–317.
- Moghe, A.K.; Gupta, B.S. Co-axial Electrospinning for Nanofiber Structures: Preparation and Applications. Polym. Rev. 2008, 48, 353–377.
- Díaz, J.E.; Fernández-Nieves, A.; Barrero, A.; Márquez, M.; Loscertales, I.G. Fabrication of structured micro and nanofibers by coaxial electrospinning. J. Phys. Conf. Ser. 2008, 127, 012008.
- Loscertales, I.G.; Barrero, A.; Guerrero, I.; Cortijo, R.; Marquez, M.; Gañán-Calvo, A.M. Micro/nano encapsulation via electrified coaxial liquid jets. Science 2002, 295, 1695–1698.
- Partheniadis, I.; Athanasiou, K.; Laidmäe, I.; Heinämäki, J.; Nikolakakis, I. Physicomechanical characterization and tablet compression of theophylline nanofibrous mats prepared by conventional and ultrasound enhanced electrospinning. Int. J. Pharm. 2022, 616, 121558.
- Weinberg, S.; King, M. Medical Fibers and Biotextiles; Academic Press: Cambridge, MA, USA, 2004; Volume 86.
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.M.; Lim, C.T.; Ramakrishna, S. Characterization of the Surface Biocompatibility of the Electrospun PCL-Collagen Nanofibers Using Fibroblasts. Biomacromolecules 2005, 6, 2583–2589.
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964.
- Salas, C. 4—Solution electrospinning of nanofibers. In Electrospun Nanofibers; Afshari, M., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 73–108.
- Sanders, E.H.; Kloefkorn, R.; Bowlin, G.L.; Simpson, D.G.; Wnek, G.E. Two-Phase Electrospinning from a Single Electrified Jet: Microencapsulation of Aqueous Reservoirs in Poly(ethylene-co-vinyl acetate) Fibers. Macromolecules 2003, 36, 3803–3805.
- Sy, J.C.; Klemm, A.S.; Shastri, V.P. Emulsion as a Means of Controlling Electrospinning of Polymers. Adv. Mater. 2009, 21, 1814–1819.
- Hu, J.; Prabhakaran, M.P.; Ding, X.; Ramakrishna, S. Emulsion electrospinning of polycaprolactone: Influence of surfactant type towards the scaffold properties. J. Biomater. Sci. Polym. Ed. 2015, 26, 57–75.
- Zamani, M.; Prabhakaran, M.P.; Ramakrishna, S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017.
- Majumder, S.; Sharif, A.; Hoque, M.E. Chapter 9—Electrospun Cellulose Acetate Nanofiber: Characterization and Applications. In Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers; Al-Oqla, F.M., Sapuan, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 139–155.
- Kulpinski, P. Cellulose nanofibers prepared by the N-methylmorpholine-N-oxide method. J. Appl. Polym. Sci. 2005, 98, 1855–1859.
- Muzzarelli, R. Natural Chelating Polymer; Belcher, R., Freisers, H., Eds.; Pergamon Press: Oxford, UK, 1973; pp. 144–176.
- Homayoni, H.; Ravandi, S.A.H.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661.
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432.
- Neamnark, A.; Rujiravanit, R.; Supaphol, P. Electrospinning of hexanoyl chitosan. Carbohydr. Polym. 2006, 66, 298–305.
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448.
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975.
- Dodero, A.; Schlatter, G.; Hébraud, A.; Vicini, S.; Castellano, M. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042.
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1754.
- Landy, D.; Mallard, I.; Ponchel, A.; Monflier, E.; Fourmentin, S. Remediation technologies using cyclodextrins: An overview. Environ. Chem. Lett. 2012, 10, 225–237.
- Djagny, K.B.; Wang, Z.; Xu, S. Gelatin: A Valuable Protein for Food and Pharmaceutical Industries: Review. Crit. Rev. Food Sci. Nutr. 2001, 41, 481–492.
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827.
- Veis, A. The Physical Chemistry of Gelatin. In International Review of Connective Tissue Research; Hall, D.A., Ed.; Elsevier: Amsterdam, The Netherlands, 1965; Volume 3, pp. 113–200.
- Dadras Chomachayi, M.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. Part A 2018, 106, 1092–1103.
- Ostrovidov, S.; Shi, X.; Zhang, L.; Liang, X.; Kim, S.B.; Fujie, T.; Ramalingam, M.; Chen, M.; Nakajima, K.; Al-Hazmi, F.; et al. Myotube formation on gelatin nanofibers—Multi-walled carbon nanotubes hybrid scaffolds. Biomaterials 2014, 35, 6268–6277.
- Topuz, F.; Uyar, T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater. Sci. Eng. C 2017, 80, 371–378.
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.-A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regenerationin vitro. J. Mater. Chem. 2009, 19, 1968–1977.
- Liu, L.; Wang, B.; Gao, Y.; Bai, T.-C. Chitosan fibers enhanced gellan gum hydrogels with superior mechanical properties and water-holding capacity. Carbohydr. Polym. 2013, 97, 152–158.
- Stijnman, A.C.; Bodnar, I.; Hans Tromp, R. Electrospinning of food-grade polysaccharides. Food Hydrocoll. 2011, 25, 1393–1398.
- Vashisth, P.; Pruthi, P.A.; Singh, R.P.; Pruthi, V. Process optimization for fabrication of gellan based electrospun nanofibers. Carbohydr. Polym. 2014, 109, 16–21.
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029.
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480.
- Adegbola, T.A.; Agboola, O.; Fayomi, O.S.I. Review of polyacrylonitrile blends and application in manufacturing technology: Recycling and environmental impact. Results Eng. 2020, 7, 100144.
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286.
- Yu, D.-G.; Branford-White, C.; Li, L.; Wu, X.-M.; Zhu, L.-M. The compatibility of acyclovir with polyacrylonitrile in the electrospun drug-loaded nanofibers. J. Appl. Polym. Sci. 2010, 117, 1509–1515.
- Balasubramanian, K.; Kodam, K.M. Encapsulation of therapeutic lavender oil in an electrolyte assisted polyacrylonitrile nanofibres for antibacterial applications. RSC Adv. 2014, 4, 54892–54901.
- Arrieta, M.P.; Leonés Gil, A.; Yusef, M.; Kenny, J.M.; Peponi, L. Electrospinning of PCL-Based Blends: Processing Optimization for Their Scalable Production. Materials 2020, 13, 3853.
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256.
- Duque Sánchez, L.; Brack, N.; Postma, A.; Pigram, P.J.; Meagher, L. Surface modification of electrospun fibres for biomedical applications: A focus on radical polymerization methods. Biomaterials 2016, 106, 24–45.
- Yew, C.H.; Azari, P.; Choi, J.R.; Muhamad, F.; Pingguan-Murphy, B. Electrospun Polycaprolactone Nanofibers as a Reaction Membrane for Lateral Flow Assay. Polymers 2018, 10, 387.
- Dimitrov, I.; Tsvetanov, C.B. 4.21—High-Molecular-Weight Poly(ethylene oxide). In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 551–569.
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30.
- Lins, L.C.; Wianny, F.; Livi, S.; Hidalgo, I.A.; Dehay, C.; Duchet-Rumeau, J.; Gérard, J.-F. Development of Bioresorbable Hydrophilic–Hydrophobic Electrospun Scaffolds for Neural Tissue Engineering. Biomacromolecules 2016, 17, 3172–3187.
- Ranjbar-Mohammadi, M.; Abbasian, M.; Mousavi, E.; Arab-Bafrani, Z. Multi-cellular tumor spheroids formation of colorectal cancer cells on Gelatin/PLCL and Collagen/PLCL nanofibrous scaffolds. Eur. Polym. J. 2019, 115, 115–124.
- Yi, B.; Shen, Y.; Tang, H.; Wang, X.; Li, B.; Zhang, Y. Stiffness of Aligned Fibers Regulates the Phenotypic Expression of Vascular Smooth Muscle Cells. ACS Appl. Mater. Interfaces 2019, 11, 6867–6880.
- Lee, J.; Guarino, V.; Gloria, A.; Ambrosio, L.; Tae, G.; Kim, Y.H.; Jung, Y.; Kim, S.H.; Kim, S.H. Regeneration of Achilles’ tendon: The role of dynamic stimulation for enhanced cell proliferation and mechanical properties. J. Biomater. Sci. Polym. Ed. 2010, 21, 1173–1190.
- Vaquette, C.; Kahn, C.; Frochot, C.; Nouvel, C.; Six, J.-L.; De Isla, N.; Luo, L.-H.; Cooper-White, J.; Rahouadj, R.; Wang, X. Aligned poly(L-lactic-co-e-caprolactone) electrospun microfibers and knitted structure: A novel composite scaffold for ligament tissue engineering. J. Biomed. Mater. Res. Part A 2010, 94A, 1270–1282.
- Xie, J.; Ihara, M.; Jung, Y.; Kwon, I.K.; Kim, S.H.; Kim, Y.H.; Matsuda, T. Mechano-active scaffold design based on microporous poly(L-lactide-co-epsilon-caprolactone) for articular cartilage tissue engineering: Dependence of porosity on compression force-applied mechanical behaviors. Tissue Eng. 2006, 12, 449–458.
- Fang, Z.; Fu, W.; Dong, Z.; Zhang, X.; Gao, B.; Guo, D.; He, H.; Wang, Y. Preparation and biocompatibility of electrospun poly(l-lactide-co-ɛ-caprolactone)/fibrinogen blended nanofibrous scaffolds. Appl. Surf. Sci. 2011, 257, 4133–4138.
- Xu, Y.; Wu, J.; Wang, H.; Li, H.; Di, N.; Song, L.; Li, S.; Li, D.; Xiang, Y.; Liu, W.; et al. Fabrication of electrospun poly(L-lactide-co-ε-caprolactone)/collagen nanoyarn network as a novel, three-dimensional, macroporous, aligned scaffold for tendon tissue engineering. Tissue Eng. C Methods 2013, 19, 925–936.
- Sperling, L.H. Introduction to Physical Polymer Science; John Wiley & Sons: Hoboken, NJ, USA, 2005.
- Kultys, A.; Rogulska, M.; Głuchowska, H. The effect of soft-segment structure on the properties of novel thermoplastic polyurethane elastomers based on an unconventional chain extender. Polym. Int. 2011, 60, 652–659.
- Zdrahala, R.J.; Zdrahala, I.J. Biomedical applications of polyurethanes: A review of past promises, present realities, and a vibrant future. J. Biomater. Appl. 1999, 14, 67–90.
- Park, J.-C.; Ito, T.; Kim, K.-O.; Kim, K.-W.; Kim, B.-S.; Khil, M.-S.; Kim, H.-Y.; Kim, I.-S. Electrospun poly(vinyl alcohol) nanofibers: Effects of degree of hydrolysis and enhanced water stability. Polym. J. 2010, 42, 273–276.
- Kamoun, E.A.; Loutfy, S.A.; Hussein, Y.; Kenawy, E.-R.S. Recent advances in PVA-polysaccharide based hydrogels and electrospun nanofibers in biomedical applications: A review. Int. J. Biol. Macromol. 2021, 187, 755–768.
- Arecchi, A.; Mannino, S.; Weiss, J. Electrospinning of poly(vinyl alcohol) nanofibers loaded with hexadecane nanodroplets. J. Food Sci. 2010, 75, N80–N88.
- Sasipriya, K.; Suriyaprabha, R.; Prabu, P.; Rajendran, V. Synthesis and characterisation of polymeric nanofibers poly (vinyl alcohol) and poly (vinyl alcohol)/silica using indigenous electrospinning set up. Mater. Res. 2013, 16, 824–830.
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706.
- Shehata, N.; Nair, R.; Boualayan, R.; Kandas, I.; Masrani, A.; Elnabawy, E.; Omran, N.; Gamal, M.; Hassanin, A.H. Stretchable nanofibers of polyvinylidenefluoride (PVDF)/thermoplastic polyurethane (TPU) nanocomposite to support piezoelectric response via mechanical elasticity. Sci. Rep. 2022, 12, 8335.
- Sukumaran, S.; Chatbouri, S.; Rouxel, D.; Tisserand, E.; Thiebaud, F.; Ben Zineb, T. Recent advances in flexible PVDF based piezoelectric polymer devices for energy harvesting applications. J. Intell. Mater. Syst. Struct. 2020, 32, 746–780.
- Houis, S.; Engelhardt, E.M.; Wurm, F.; Gries, T. Application of Polyvinylidene Fluoride (PVDF) as a Biomaterial in Medical Textiles. In Medical and Healthcare Textiles; Anand, S.C., Kennedy, J.F., Miraftab, M., Rajendran, S., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 342–352.
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric biomaterials for sensors and actuators. Adv. Mater. 2019, 31, 1802084.
- Yuan, H.; Lei, T.; Qin, Y.; He, J.-H.; Yang, R. Design and application of piezoelectric biomaterials. J. Phys. D Appl. Phys. 2019, 52, 194002.
- Soluble polyvinylpyrrolidone (Povidone). In Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Bühler, V. (Ed.) Springer: Berlin/Heidelberg, Germany, 2005; pp. 5–124.
- Liu, X.; Xu, Y.; Wu, Z.; Chen, H. Poly(N-vinylpyrrolidone)-Modified Surfaces for Biomedical Applications. Macromol. Biosci. 2013, 13, 147–154.
- Wang, C.; Ma, C.; Wu, Z.; Liang, H.; Yan, P.; Song, J.; Ma, N.; Zhao, Q. Enhanced bioavailability and anticancer effect of curcumin-loaded electrospun nanofiber: In vitro and in vivo study. Nanoscale Res. Lett. 2015, 10, 439.
- Yoo, H.J.; Kim, H.D. Characteristics of waterborne polyurethane/poly (N-vinylpyrrolidone) composite films for wound-healing dressings. J. Appl. Polym. Sci. 2008, 107, 331–338.
- Rahmani, F.; Ziyadi, H.; Baghali, M.; Luo, H.; Ramakrishna, S. Electrospun PVP/PVA Nanofiber Mat as a Novel Potential Transdermal Drug-Delivery System for Buprenorphine: A Solution Needed for Pain Management. Appl. Sci. 2021, 11, 2779.
- Kaplan, D.; Adams, W.W.; Farmer, B.; Viney, C. Silk Polymers: Materials Science and Biotechnology; ACS Publications: Washington, DC, USA, 1993.
- Park, W.H.; Ha, W.S.; Ito, H.; Miyamoto, T.; Inagaki, H.; Noishiki, Y. Relationships between antithrombogenicity and surface free energy of regenerated silk fibroin films. Fibers Polym. 2001, 2, 58–63.
- Santin, M.; Motta, A.; Freddi, G.; Cannas, M. In vitro evaluation of the inflammatory potential of the silk fibroin. J. Biomed. Mater. Res. 1999, 46, 382–389.
- Yang, J.-M.; Wang, N.-C.; Chiu, H.-C. Preparation and characterization of poly(vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J. Membr. Sci. 2014, 457, 139–148.
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374.
- Kyzioł, A.; Michna, J.; Moreno, I.; Gamez, E.; Irusta, S. Preparation and characterization of electrospun alginate nanofibers loaded with ciprofloxacin hydrochloride. Eur. Polym. J. 2017, 96, 350–360.
- Pérez-Guzmán, C.J.; Castro-Muñoz, R. A Review of Zein as a Potential Biopolymer for Tissue Engineering and Nanotechnological Applications. Processes 2020, 8, 1376.
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Effect of α-tocopherol antioxidant on rheological and physicochemical properties of chitosan/zein edible films. LWT 2020, 118, 108799.
- Khatri, M.; Khatri, Z.; El-Ghazali, S.; Hussain, N.; Qureshi, U.A.; Kobayashi, S.; Ahmed, F.; Kim, I.S. Zein nanofibers via deep eutectic solvent electrospinning: Tunable morphology with super hydrophilic properties. Sci. Rep. 2020, 10, 15307.
This entry is offline, you can click here to edit this entry!
