Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Spirulina is a kind of blue-green algae (BGA) that is multicellular, filamentous, and prokaryotic. It is also known as a cyanobacterium. It is classified within the phylum known as blue-green algae. Despite the fact that it includes a high concentration of nutrients, such as proteins, vitamins, minerals, and fatty acids—in particular, the necessary omega-3 fatty acids and omega-6 fatty acids—the percentage of total fat and cholesterol that can be found in these algae is substantially lower when compared to other food sources.
- spirulina algae
- chemical composition
- health and nutritional value
1. Introduction
Spirulina algae, also known as Arthrospira platensis, are members of the class of cyanobacteria (also named blue-green algae) that are classified under the phylum of multicellular organisms. These filaments are unbranched and spiral in shape. Algae are a diverse group of aquatic organisms that have the ability to conduct photosynthesis. In subtropical and tropical climates, such as Hawaii, Mexico, Asia, and Central Africa, they flourish naturally in water tanks that contain high levels of salt and alkaline. GRAS stands for “generally regarded as safe,” which is the designation that the Food and Drug Administration (FDA) has bestowed upon it. Research on humans in clinical trials, as well as studies on animals carried out in the most recent decade, provide credence to this assertion. A. platensis, A. maxima, and A. fusiformis are three of the species of spirulina that have been put to use in food, and have been the subject of a significant amount of research [1][2][3][4].
Spirulina algae have high nutritional value. As a result of their high protein content (60–70% on a dry weight basis), vitamins, minerals, essential fatty acids, and other nutrients, the FDA has designated them as the ideal food for mankind and a “super food,” containing high concentrations of beta(β)-carotene, vitamin B12, iron, trace elements, and the extremely rare essential gamma(γ)-linolenic acid. The Food and Agriculture Organization (FAO) of the United Nations have referred to spirulina as a “highly digestible protein product,” and the US space agency has utilized it as a dietary supplement for astronauts. Because of this, spirulina deserves the title of “the food of the future” more than any other food on Earth [1][2].
It has been demonstrated that spirulina is both biologically and economically significant due to the numerous applications that have been developed for it in the food, pharmaceutical, biofuel, cosmetics, and agricultural industries. These algae are readily accessible for purchase and have a significant geographic distribution. This is because the manufacturers want to obtain the biomass of spirulina in order to make use of its important biologically active compounds, such as phycocyanins, phenols, polysaccharides, polyunsaturated fatty acids (PUFAs), carotenoids, vitamins, and sterols. This is why there is such a high demand for spirulina. The majority of these compounds play an important therapeutic role in the treatment of cardiovascular diseases (CVDs), high cholesterol, high blood sugar, obesity, high blood pressure, tumors, and inflammatory diseases. In addition to bolstering the immune system, the presence of these compounds is associated with a reduced risk of developing neurodegenerative conditions, such as Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis, in particular. Spirulina is regarded as a natural medicine and is utilized in the manufacturing of functional foods and nutritional supplements all over the world due to the qualities that have been described [5][6].
Spirulina algae can be produced in the form of powder, liquid, oil, tablets, or capsules, and are used in many food industries, including the manufacture of sweets, snacks, and pastries. This helps the market meet the demand for variety while also providing highly nutritious food that can aid in the feeding of children and the fight against malnutrition [7]. In addition to the introduction of spirulina in the production of functional beverages, such as fruit juices, which have gained a great deal of relevance in terms of health, it is also employed in the production of dairy products, pasta, oil derivatives, and nutritional supplements [8][9][10]. In addition to being used as a coloring agent in the food industry, spirulina has a wide range of uses in the areas of human nutrition, animal feed, and fish feed [11][12][13].
The production of spirulina algae, which are a rich source of protein, has increased in recent years. This coincides with an increase in the demand for protein, which has led to the development of the industry. Because of this, food companies have begun marketing proteins derived from a range of sources, including those derived from animals, plants, single-celled organisms, and spirulina. Pasta, sushi, and jerky are just a few examples of the new food products that have been produced for consumers that are based on spirulina [14][15].
2. A Brief Overview on Spirulina as Important Algae for Human Food and Health
The term “algae” refers to a wide collection of organisms that produce their own food via the process of photosynthesis and may be found in a variety of habitats, including marine and freshwater environments [3]. They are found in almost every part of the world and may be divided into two categories. Microalgae are the most basic and fundamental members of the plant kingdom. The bulk of their cells are rather thin, measuring between 3 and 20 µm, and some species form simple colonies. Macroalgae are typically multicellular, expand at a quick rate, and can reach widths of up to 20 m. When compared to the growth rates of terrestrial plants, the rates of growth of macroalgae are significantly higher. Production of macroalgae in maritime habitats, also known as seaweed, does not need the usage of arable land or fertilizer and can take place without either of those factors being present. Seaweeds have the capacity to generate more biomass per hectare than vascular plants do, develop at a far faster rate, and make use of the light energy and carbon dioxide that is taken in from the environment. In the field of applied botany, the tiny algae known as cyanobacteria were once known as cyanophyceae. Cyanobacteria are some of the earth’s oldest primitives. They are one of the prokaryotes that have certain properties in common with plants, such as the capacity to carry out photosynthesis, and their cytoskeleton is similar (phototrophic nutrition). The cellular forms of cyanobacteria have undergone several transformations during the course of their evolution, ranging from unicellular to multicellular structures. They can be found in ecosystems containing fresh water, marine life, and terrestrial life, as well as certain severe or harsh habitats, such as hot springs, dry soils, some saline environments, and glaciers [3][16][17][18].
Arthrospira platensis is the species of spirulina that is multicellular, filamentous, heterogeneous, non-branching, and does not fix nitrogen. It is also capable of photosynthesis and the production of chemical compounds that are necessary for existence. It is grown in liquid farms that are located within open ponds, and flourishes naturally in brackish waters, salt lakes, and warm conditions that are rich in bicarbonate and carbonate [19][20][21][22][23]. In Iraq, many species of spirulina, such as A. jenner, were discovered, identified as novel algae, and listed in the inventory of Iraq’s algal flora [24][25].
Before 1962, spirulina was considered to be a type of algae. However, in that year, it was reclassified as a member of the prokaryotic kingdom, and the name “cyanobacteria” was suggested for it [18][26]. Different-sized filaments or spiral trichomes can be produced by organisms belonging to the genus Arthrospira. Spirulina may fold and bend to varying degrees, taking on shapes that range from a tightly coiled form to a shape that is straight and unwound. Solitary in nature, filaments reproduce by a process known as binary fission. The lengths of the filaments typically range from 2 to 12 µm but can go as high as 16 µm at times [27][28]. The diameter of the thread ranges anywhere from 3 to 12 µm, and the cells that make up the filament contain gas vacuoles that aid in floating [19][29][30].
3. Nutritional and Biochemical Components of Spirulina
Food is the primary means through which the body receives the myriad of vital nutrients that are required for development, the performance of essential biological activities, and the preservation of overall health. On the one hand, considering that our bodies are unable to produce some nutrients, it is necessary to receive them through this diet. On the other hand, several diseases have been related to an imbalance in the human diet, which can be caused by the presence of certain unsuitable nutritional components or the body’s incapacity to absorb them [31]. The overall composition of spirulina changes depending on the source of the algae used to cultivate it, the environmental conditions of the manufacturing facility, and the season of the year. Proteins make up between 55% and 70% of the body of spirulina, while carbohydrates make up between 15% and 25%, fats make up between 6% and 8%, minerals make up between 7 and 13%, moisture (dried algae) makes up between 3% and 7%, and dietary fibers make up between 8% and 10% [32]. Figure 1 presents a description of the components that make up spirulina. The proportion of PUFAs is between 1.5% and 2% of the total fat content, and it is rich in linolenic acid, which accounts for 36% of the total PUFAs, as well as vitamins (B1, B2, B3, B6, B9, B12, C, D, and E) and minerals (K, Ca, Cr, Cu, Fe, Mg, Mn, P, Se, Na, and Zn), as well as the pigments (chlorophyll A, xanthophylls, β-carotene, echinenone, myxoxanthophyll, zeaxanthin, canthaxanthin, diatoxanthin, 3-hydroxychininone, β-cryptoxanthin oscillaxanthin, phycobiliproteins, C-phycocyanin, allophycocyanin) and enzymes (such as lipase) [33]. The components of spirulina’s chemical makeup are summarized in Table 1.
Table 1. The value of proximate composition of spirulina from different reported research.
| Proximate Composition (%) | Food Energy | References | |||||
|---|---|---|---|---|---|---|---|
| Moisture | Fat/Lipid | Protein | Ash | Fiber | Carbohydrate | ||
| 4–5 | 4–7 | 65–72 | 6–12% | 3–7 | 15–25 | 2.90 cal/g | [34] |
| 3–7 | 6–8 | 55–70 | 7–13 | 8–10 | 15–25 | – | [32] |
| 5.37 | 7.19 | 61.57 | 7.10 | 7.93 | 16.21 | – | [35] |
| 5.45–9.92 | 6.61–6.84 | 52.85–65.00 | 9.55–9.93 | 9.79–11.37 | 15.29–13.62 | 329.89–379.58 | [36] |
| 5.27 | 1.27 | 71.90 | 3.50 | 9.70 | 13.63 | 353.55 | [37] |
| 4.74 | 6.93 | 62.84 | 7.47 | 8.12 | – | – | [38] |
| – | 7.16 | 52.95 | – | – | 13.20 | – | [39] |
| 1 | 6 | 63 | 8 | – | 22 | – | [40] |
| – | 4 | 65 | 3 | 3 | 19 | – | [41] |
| 4–6 | 5–7 | 55–70 | 3–6 | 5–7 | – | – | |
| 6 | 6 | 61 | 9 | – | 14 | – | |
| 9 | 7 | 60 | 11 | – | – | – | |
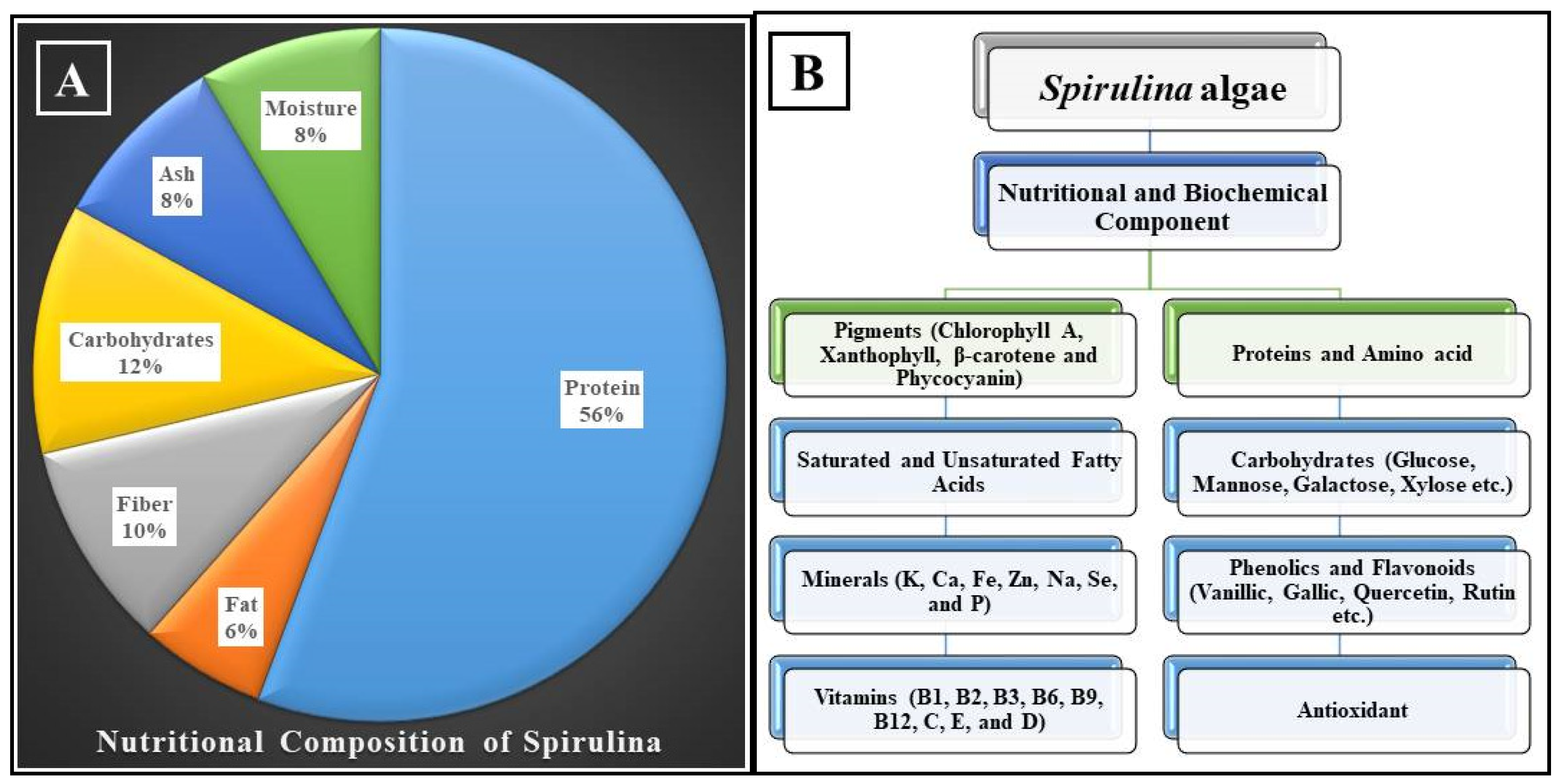
Figure 1. (A) Nutritional composition and (B) biochemical components of spirulina [36].
3.1. Carbohydrates
According to the findings of various studies, the proportion of carbohydrates present in spirulina spp. is around 13.6% [42][43]. On the other hand, a number of additional studies came to the conclusion that the total carbohydrate content of spirulina ranged from 15% to 25% dry weight [32][33][42][44][45][46]. There is no cellulose present in spirulina algae’s carbohydrates; instead, they are made up of a variety of sugars, such as glucose, mannose, galactose, and xylose, in addition to glycogen. As a result, the carbohydrates included in spirulina are simple to digest, as well as nutrient-dense, and may be consumed by elderly individuals and those who have intestinal malabsorption. In addition to that, it has a polysaccharide with high molecular weight known as immolina. Rhamnose is the primary component in it, accounting for around 52.3% of the total sugars generated by spirulina. In another variety, rhamnose accounts for roughly 49.7% of the total sugars produced. Spirulina has a biomass of 1.22 g/L, its polysaccharide content is 2.590% of its biomass, and the total sugars that it contains are 17.275% of its polysaccharides [6][43][47][48][49]. Polymers, such as glucosamine (1.9%), rhamnosamine (9.7%), and glycogen (0.5%), as well as small amounts of glucose, fructose, sucrose, glycerine, mannitol, and sorbitol, are the primary components of virtually all absorbable carbohydrates. Spirulina has sugars in its cell wall that are analogous to the sugars found in the cell walls of Gram-negative bacteria. These are composed of glucosamine, muramic acid, and glucosamine that have bound to peptides. Due to the fact that these cell walls are relatively thin, digestive enzymes are able to access the contents of the cell with relative ease [42]. Of the various culture media that are utilized during the production of spirulina, each has an impact on the total amount of carbohydrates that are produced. According to the findings of Madkour et al. [39], the percentage of carbohydrate content in spirulina algae grown in low-cost culture media varied depending on the type of nitrogen source present in the culture medium. To accomplish this, all of the nutrients found in the standard medium are swapped out for more affordable and readily available commercial chemicals and fertilizers in the region. The percentage of carbohydrates present in the medium with the standard nitrogen source was 13.20%, but this percentage increased to 16.01% when the nitrogen source was replaced with a medium containing urea. In the ammonium nitrate (NH4NO3) medium, the concentration of carbohydrates rose to 24.50% on a dry weight basis; however, other researchers discovered that the amount of carbohydrates varied depending on the region of production and the kind of product being made [32].
3.2. Lipids/Fats and Fatty Acids
According to the findings of several researchers, the lipid content of S. platensis ranges from 5% to 10% of the dry weight. Other research that used more effective extraction techniques found that the percentage was greater than 11%. In most cases, it will contain fats that are necessary for human survival, and free fatty acids will make up between 70% and 80% of the total fat. These total lipids may be divided into a saponified fraction that makes up 83% of the total and an unsaponifiable fraction that makes up 17%, with the unsaponifiable fraction mostly consisting of paraffin, pigments, terpene alcohols, and sterols. Omega-6 fatty acids make up the majority of the total fat, and there is just a trace quantity of cholesterol (less than 0.1 mg/100 g dry mass) present [33][42]. Adults need 1–2% of their total energy intake to come from essential fatty acids, whereas children need 3% of their total energy intake [50].
The location of the closest polyunsaturated point in the MTG is used to describe the optimal omega-6 to omega-3 ratio that some nutritionists advocate, which falls between 4 and 5 [31][51]. It was discovered that the total fatty acid concentration of A. platensis is 81.2 mg/g on a dry weight basis, which demonstrates that spirulina is an excellent source of fatty acids [52]. However, Sharoba [38] discovered that the proportion of total saturated fatty acids was 44.21 mg/100 g, but the proportion of total essential unsaturated fatty acids was 55.79 mg/100 g. When looking at the nutritional value of spirulina, researchers found that it has a significant amount of palmitic acid (16:0), which makes up more than 60% of the lipids in S. maxima and 25% in S. platensis, respectively. While the proportion of saturated palmitic acid in the total fatty acids was 25.8%, the percentage of γ-linolenic acid in the total fatty acids was 40.1%. Spirulina is an excellent dietary supplement for essential fatty acid deficits as a result [42].
Spirulina was discovered to have a significant quantity of PUFAs, with levels ranging from 1.5% to 2.0% fat. This has piqued the curiosity of many researchers, who have been doing studies on PUFAs to determine how much of this nutrient is contained in spirulina [23]. According to the findings of another study, PUFAs made about 30% of the total fats [6], while other researchers reported that the proportion of these fatty acids ranged between 19.4% and 21.9% of the total fatty acids [53]. Its primary fatty acid, 15,12.9-octadecatrienoic acid, accounted for 10.1% of its total fatty acid content, whereas the omega-3 content accounted for less than 1% of its total fatty acid content. Additionally, it had some omega-6 type fatty acids. In addition to this, a significant amount of saturated hexadecanoic acid was discovered (37.6%). The concentration of monounsaturated fatty acids (MUFAs) was low, with the octadec-9-enoic acid (18:1) omega-9 type falling below 2.0%. The quantity of γ-linolenic acid, which came in at 16 mg, had the greatest content, followed by palmitic acid, which had the highest percentage (23%), while myristic acid had the lowest percentage (0.2%) [52].
According to Matos et al. [53], the amount of fatty acids in spirulina algae might vary depending on a variety of parameters, including the growing circumstances and development stage at the time of harvest. The total fatty acid content was estimated to be 4.25 mg/100 g, and it was discovered to include sapienic acid at a level of 2.25 mg/100 g, linoleic acid at a level of 16.7%, and γ-linolenic acid at a level of 14% [51]. According to the findings of Alyasiri et al. [54], one gram of spirulina has a high concentration of linolenic acid of the omega-6 type; specifically, the concentration was 29.1 mg/g, which corresponds to a rate of 2.91%. Additionally, it has PUFAs, which are saturated with 18 carbon atoms and include omega-6. When it comes to the most significant biologically active compounds found in spirulina, phytol had the highest percentage (100%), followed by monolinoleoylglyceroltrimethylsilylether-1b (71.31%), steroid and cholestan-3-ol (2-methylene-3β, 5α) (54.62%), and 9, 12, 15-octadecatrienoic acid, 2, 3-dihydroxypropyl ester (28.21%), hexadecanoic acid methyl ester (23.23%), and methenamine (23.21%) [55]. According to Legezynska et al. (2014), spirulina algae are one of the primary sources of omega-3 fatty acids that fish feed on. Examples of these fatty acids are docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). As a result, a higher percentage of these essential fatty acids might be found in fish oils [56]. Linoleic acid, which belongs to the omega-6 group, and alpha (α)-linolenic acid, which belongs to the omega-3 group, is found in the fats of marine algae, suspended algae, and fish oils, respectively. EPA, DHA, α-linolenic acid, and docosapentaenoic acid are the four essential fatty acids (omega-3) that are considered to be of the utmost importance [57]. According to the research conducted by Liestianty et al. [52], the fatty acids contained in spirulina include myristic, heptadecanoic, stearic, oleic, palmitoleic, omega-3, omega-6, linoleic acid, and palmitic acid. Omega-6 kinds, the most significant of which are palmitoleic, oleic, linoleic, and γ-linolenic, and omega-3 types, including α-linoleic acid, are among the most essential types that may be found [38]. Linolenic acid, stearidonic acid, EPA, DHA, and arachidonic acid are found in high concentrations in it [23]. The omega-6 family, which includes γ-linolenic acid and arachidonic acid, and the omega-3 family, which includes EPA and DHA, are the most essential long-chain PUFAs that algae can produce [6]. Utilizing gas chromatography–mass spectrometry (GC-MS) and high-performance liquid chromatography (HPLC), Al-Dhabi and Valan Arasu [51] were able to identify PUFAs in 37 different commercialized spirulina species. Myristic acid, stearic acid, and eicosadienoic acid were identified as the three saturated fatty acids that were present in the spirulina samples. It was found that ten of the unsaturated fatty acids in the spirulina samples were substantially different from one another.
The accumulation of toxic compounds in fish, as well as the odor, strange taste, and oxidative instability of the oils extracted from fish, has a negative impact on the total dependence on the synthesis of long-chain PUFAs (especially omega-3 type) from fish oil. This has a negative impact on the total dependence on the synthesis of long-chain PUFAs from fish oil. As a result, the focus shifted toward the possibility of employing spirulina algae in a commercial environment as a different source to produce these fatty acids [56].
Spirulina algae are a potential source of polyunsaturated fatty acids (PUFAs). Essential fatty acids, such as omega-3 and omega-6, are unable to be produced by humans and, as a result, must be received through the consumption of food. They play a significant role in preserving health and warding off disease. Even though the human gut microbiota is capable of synthesizing long-chain fatty acids, such as linoleic and α-linolenic acids, the synthesis of these acids is controlled by various variables, which makes the consumption of these fatty acids vital for the maintenance of good health [6]. Because it is not commonly found in foods that people eat on a regular basis, despite it having a high nutritional value, the presence of -linolenic acid is interesting. This acid is typically generated in humans from γ-linolenic acid (18:2 omega-6), which comes from vegetable sources [42].
Spirulina is the only food source that contains large amounts of essential fatty acids, especially γ-linolenic acid, which is an omega-6 type that helps regulate all hormones and has anti-inflammatory properties. Comparatively, breast milk is the only food source that contains large amounts of essential fatty acids [19][39][54]. The other supply comes from the oil that is derived from borage, black currant, and evening primrose seeds. In comparison, an evening primrose oil intake of 500 mg has just 45 mg of γ-linolenic acid, whereas 10 g of spirulina has 135 mg of γ-linolenic acid. Comparatively, evening primrose oil only contains 9% linoleic acid, whereas the lipids of spirulina contain around 20–25% of γ-linolenic acid [58].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27175584
References
- Carlson, S. Spirulina platensis (Conventional and Organic), Spirulina, Organic Spirulina, or Arthrospira Platensis; Division of Biotechnology and Gras Notice Review, Office of Food Additive Safety-CFSAN: Dauphin Island, AL, USA, 2011; p. 36.
- Haoujar, I.; Haoujar, M.; Altemimi, A.B.; Essafi, A.; Cacciola, F. Nutritional, sustainable source of aqua feed and food from microalgae: A mini review. Int. Aquat. Res. 2022, 14, 1–9.
- Singh, S.; Verma, D.K.; Thakur, M.; Tripathy, S.; Patel, A.R.; Shah, N.; Utama, G.L.; Srivastav, P.P.; Benavente-Valdés, J.R.; Chávez-González, M.L.; et al. Supercritical Fluid Extraction (SCFE) as Green Extraction Technology for High-value Metabolites of Algae, Its Potential Trends in Food and Human Health. Food Res. Int. 2021, 150 Pt A, 110746.
- Nascimento, R.Q.; Deamici, K.M.; Tavares, P.P.L.G.; de Andrade, R.B.; Guimarães, L.C.; Costa, J.A.V.; Guedes, K.M.; Druzian, J.I.; de Souza, C.O. Improving water kefir nutritional quality via addition of viable Spirulina biomass. Bioresour. Technol. Rep. 2022, 17, 100914.
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676.
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods. Nutraceuticals Food Suppl. 2018, 6, 45–58.
- Gogna, S.; Kaur, J.; Sharma, K.; Prasad, R.; Singh, J.; Bhadariya, V.; Kumar, P.; Jarial, S. Spirulina-An Edible Cyanobacterium with Potential Therapeutic Health Benefits and Toxicological Consequences. J. Am. Nutr. Assoc. 2022; in press.
- El-Beltagi, H.S.; Dhawi, F.; Ashoush, I.S.; Ramadan, K. Antioxidant, anti-cancer and ameliorative activities of Spirulina platensis and pomegranate juice against hepatic damage induced by CCl4. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1941–1956.
- Manjula, R.; Vijayavahini, R.; Lakshmi, T.S. Formulation and quality evaluation of spirulina incorporated ready to serve (RTS) functional beverage. Int. J. Multidiscip. Res. Arts Sci. Commer. 2021, 1, 29–35.
- Aljobair, M.O.; Albaridi, N.A.; Alkuraieef, A.N.; AlKehayez, N.M. Physicochemical properties, nutritional value, and sensory attributes of a nectar developed using date palm puree and spirulina. Int. J. Food Prop. 2021, 24, 845–858.
- Shahidi, F.; Alasalvar, C. Handbook of Functional Beverages and Human Health; CRC Press: Boca Raton, FL, USA, 2016; Volume 11, 886p.
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Rev. Foods 2017, 6, 33.
- Tavakoli, M.; Habibi Najafi, M.B.; Mohebbi, M. Effect of The Milk Fat Content and Starter Culture Selection on Proteolysis and Antioxidant Activity of Probiotic Yogurt. Heliyon 2019, 5, e01204.
- Li, D.M.; Qi, Y.Z. Spirulina industry in China: Present status and future prospects. J. Appl. Phycol. 1997, 9, 25–28.
- Grahl, S.; Strack, M.; Weinrich, R.; Mörlein, D. Consumer-Oriented Product Development: The Conceptualization of Novel Food Products Based on Spirulina (Arthrospira platensis) and Resulting Consumer Expectations. J. Food Qual. 2018, 2018, 1919482.
- Fogg, G.E.; Thake, B. Algal Cultures and Phytoplankton Ecology; University of Wisconsin Press: Madison, WI, USA, 1987.
- Schirrmeister, B.E.; Antonelli, A.; Bagheri, H.C. The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 2011, 11, 45.
- Siva Kiran, R.R.; Madhu, G.M.; Satyanarayana, S.V. Spirulina in combating protein energy malnutrition (PEM) and protein energy wasting (PEW)—A review. J. Nutr. Res. 2015, 1, 62–79.
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578.
- Vonshak, A. Spirulina: Growth, physiology and biochemistry. In Spirulina platensis (Arthrospira): Physiology, Cell Biology and Biotechnology; Taylor and Francis Ltd.: London, UK, 1997; pp. 43–65.
- Pelizer, L.H.; Danesi, E.D.G.; Rangel, C.O.; Sassano, C.E.; Carvalho, J.C.M.; Sato, S.; Moraes, I.O. Influence of inoculum age and concentration in Spirulina platensis cultivation. J. Food Eng. 2003, 56, 371–375.
- Costa, J.A.V.; Colla, L.M.; Filho, P.D. Spirulina platensis growth in open raceway ponds using fresh water supplemented with carbon, nitrogen and metal ions. Z. Nat. C -A J. Biosci. 2003, 58, 76–80.
- Usharani, G.; Saranraj, P.; Kanchana, D. Spirulina cultivation: A review. Int. J. Pharm. Biol. Arch. 2012, 3, 1327–1341.
- Maulood, B.K.; Hassan, F.M.; Al-Lami, A.A.; Toma, J.J.; Ismail, A.M. Checklist of Algal Flora in Iraq; Ministry of Environment: Baghdad, Iraq, 2013.
- Al-Yassiry, T.M.H. Ecological Assessment of the Sewage in The City of Hilla/Iraq Using the Canadian Model and the Study of Phytoplankton. Master’s Thesis, University of Babylon, Hillah, Iraq, 2014.
- Stanier, R.Y.; Van Niel, C.B. The concept of a bacterium. Arch. Mikrobiol. 1962, 42, 17–35.
- Gershwin, M.E.; Belay, A. (Eds.) Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: London, UK; New York, NY, USA, 2007.
- Heinsoo, D. Cultivation of Spirulina on Conventional and Urine Based Medium in a Household Scale System. Master’s Thesis, KTH School of Biotechnology, Stockholm, Sweden, 2014; 46p.
- Tomaselli, L. Morphology, ultrastructure and taxonomy of Arthrospira (Spirulina) maxima and Arthrospira (Spirulina) platensis. In Spirulina platensis (Arthrospira): Physiology, Cell Biology and Biotechnology; Vonshak, A., Ed.; Taylor and Francis: London, UK, 1997; pp. 1–15.
- Belay, A.; Kato, T.; Ota, Y. Spirulina (Arthrospira): Potential application as an animal feed supplement. J. Appl. Phycol. 1996, 8, 303–311.
- Choopani, A.; Poorsoltan, M.; Fazilati1, M.; Latifi, A.M.; Salavati, H. Spirulina a source of gamma-linoleic acid. J. Appl. Biotechnol. Rep. 2016, 3, 483.
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Spirulina platensis, a super food? J. Cell. Biotechnol. 2019, 5, 43–54.
- Hosseini, S.M.; Shahbazizadeh, S.; Khosravi-Darani, K.; Reza Mozafari, M. Spirulina paltensis: Food and function. Curr. Nutr. Food Sci. 2013, 9, 189–193.
- El Nakib, D.M.; Ibrahim, M.M.; Mahmoud, N.S.; Abd El Rahman, E.N.; Ghaly, A.E. Incorporation of Spirulina (Athrospira platensis) in traditional Egyptian cookies as a source of natural bioactive molecules and functional ingredients: Preparation and sensory evaluation of nutrition snack for school children. Eur. J. Nutr. Food Saf. 2019, 9, 372–397.
- Bahlol, H.E.M. Utilization of Sprulina Algae to Improve the Nutritional Value of Kiwifruits and Cantaloupe Nectar Blends. Ann. Agric. Sci. Moshtohor 2018, 56, 315–324.
- Michael, A.; Kyewalyanga, M.S.; Mtolera, M.S.; Lugomela, C.V. Antioxidants activity of the cyanobacterium, Arthrospira (Spirulina) fusiformis cultivated in a low-cost medium. Afr. J. Food Sci. 2018, 12, 188–195.
- Saharan, V.; Jood, S. Nutritional composition of Spirulina platensis powder and its acceptability in food products. Int. J. Adv. Res. 2017, 5, 2295–2300.
- Sharoba, A.M. Nutritional value of spirulina and its use in the preparation of some complementary baby food formulas. J. Agroaliment. Process. Technol. 2014, 20, 330–350.
- Madkour, F.F.; Kamil, A.E.W.; Nasr, H.S. Production and nutritive value of Spirulina platensis in reduced cost media. Egypt. J. Aquat. Res. 2012, 38, 51–57.
- Malik, P. Utilization of Spirulina Powder for Enrichment of Ice Cream and Yoghurt. Master’s Thesis, Karnataka Veterinary, Animal and Fisheries Sciences University, Bida, India, 2011; 151p.
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish; FAO Fisheries and Aquaculture Circular No. 1034; FAO: Rome, Italy, 2008.
- Falquet, J.; Hurni, J.P. The Nutritional Aspects of Spirulina. Antenna Foundation. 1997. Available online: https://www.antenna.ch/wp-content/uploads/2017/03/AspectNut_UK (accessed on 10 August 2022).
- Seyidoglu, N.; Inan, S.; Aydin, C. A prominent superfood: Spirulina platensis. In Superfood and Functional Food the Development of Superfoods and Their Roles as Medicine; IntechOpen: London, UK, 2017; Volume 22, Chapter 1; pp. 1–27.
- Ravi, M.; De, S.L.; Azharuddin, S.; Paul, S.F. The beneficial effects of Spirulina focusing on its immunomodulatory and antioxidant properties. Nutr. Diet. Suppl. 2010, 2, 73–83.
- Sotiroudis, T.G.; Sotiroudis, G.T. Health aspects of Spirulina (Arthrospira) microalga food supplement. J. Serb. Chem. Soc. 2013, 78, 395–405.
- Jamil, A.B.M.R.; Akanda, M.R.; Rahman, M.M.; Hossain, M.A.; Islam, M.S. Prebiotic competence of spirulina on the production performance of broiler chickens. J. Adv. Vet. Anim. Res. 2015, 2, 304–309.
- Walter, P. Effects of vegetarian diets on aging and longevity. Nutr. Rev. 1997, 55, S61–S65.
- Mishra, P.; Singh, V.P.; Prasad, S.M. Spirulina and its Nutritional Importance: A Possible Approach for Development of Functional Food. Biochem. Pharmacol. 2014, 3, e171.
- Rachidi, F.; Benhima, R.; Kasmi, Y.; Sbabou, L.; Arroussi, H.E. Evaluation of microalgae polysaccharides as biostimulants of tomato plant defense using metabolomics and biochemical approaches. Sci. Rep. 2021, 11, 930.
- Pascaud, M.; Brouard, C. Acides gras polyinsaturés essentiels ω6 et ω3. Besoins nutritionnels, équilibres alimentaires. Cah. Nutr. Diététique 1991, 26, 185–190.
- Al-Dhabi, N.A.; Valan Arasu, M. Quantification of phytochemicals from commercial Spirulina products and their antioxidant activities. Evid. -Based Complement. Altern. Med. 2016, 2016, 7631864.
- Liestianty, D.; Rodianawati, I.; Andi Arfah, R.; Asma Assa, A.; Patimah; Sundari; Muliadi. Nutritional analysis of spirulina sp. to promote as superfood candidate. In Proceedings of the 13th Joint Conference on Chemistry (13th JCC), Semarang, Indonesia, 7–8 September 2018; pp. 1–6.
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312.
- Alyasiri, T.; Alchalabi, S.; AlMayaly, I. In vitro and In vivo antioxidant effect of Spirulina platensis against Lead induced toxicity in rats. Asian J. Agric. Biol. 2018, 6, 66–77.
- Gheda, S.F.; Abo-Shady, A.M.; Abdel-Karim, O.H.; Ismail, G.A. Antioxidant and Antihyperglycemic Activity of Arthrospira platensis (Spirulina platensis) Methanolic Extract: In vitro and in vivo Study. Egypt. J. Bot. 2021, 61, 71–93.
- Viso, A.C.; Marty, J.C. Fatty-acids from 28 marine microalgae. Phytochemistry 1993, 34, 1521–1533.
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231.
- Henrikson, R. A nutrient rich super food for super health. In Earth Food Spirulina; Ronore Enterprises, Inc.: Hana, HI, USA, 2009; pp. 25–41.
This entry is offline, you can click here to edit this entry!
