ABA controls multiple plant physiological and biochemical processes. Here we have highlighted the role of this hormone in the regulation of plant WUE and reviewed promising biotechnogical approches to confer drought resistance and improve crop WUE.
- ABA
- drought
- hydraulic conductivity
- WUE
- xylem embolism
- aba receptors
1. Introduction
The phytohormone abscisic acid (ABA) is a fundamental regulator of the morpho-physiological response of plants during drought stress. The central and best-known plant response to ABA during drought is the closure of stomata in seed plants. The effects of ABA on shoot and root growth can be positive or negative depending on its concentration and the plant species [1]. Increased levels of endogenous ABA also play key roles in down-regulating leaf hydraulic conductance (which is proposed to further induce stomatal closure) [1][2][3] and mesophyll conductances [4][5][6], as well as up-regulating the cuticular wax formation (which results in a thicker, less permeable cuticle) [7][8]. Finally, ABA is a critical messenger produced in response to water deficit that promotes a variety of biochemical responses in different plant tissues [9]. Due to the ABA’s key roles in the physiology of plants during drought, several promising approaches to manipulate ABA biosynthesis and signaling (i.e., either genetically or through chemical intervention with agonists) have been explored aiming at conferring drought tolerance to major crop species and improving water use efficiency (WUE, i.e., the ratio of photosynthesis to water loss through foliar transpiration). In this review, we cover the progress made in the last decade in our understanding of how ABA signaling regulates plant physiology and biochemistry during drought. Special attention has been paid to (i) the ABA biosynthesis within the plant, (ii) the impacts of ABA on stomatal aperture and xylem embolism, (iii) the regulation of primary and secondary metabolisms by ABA, and (iv) the potential applications of approaches modulating ABA levels and perception to improve WUE and drought tolerance in crop species.
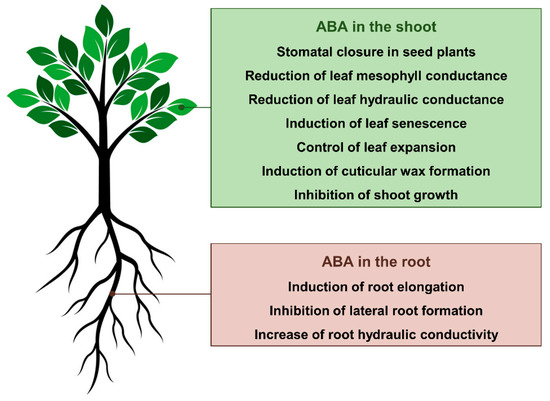
Figure 1. Current understanding of how abscisic acid (ABA) regulates plant form and function during drought.
2. Drought-Induced Biosynthesis of ABA
Given the key role played by ABA in enhancing plant survival during drought, a comprehensive understanding of how the biosynthesis of ABA occurs, how it is regulated within the plant, and where it mostly takes place is of major importance. In recent years, the pathway by which ABA is synthesized in plants has been extensively investigated through the use of mutants deficient in the synthesis and perception of ABA [10][11][12][13][14]. It has been demonstrated that the biosynthetic pathway for ABA accumulation begins in the chloroplasts with the hydroxylation of β-carotene to zeaxanthin, which is then converted to violaxanthin through the xanthophyll cycle. Next, neoxanthin synthase converts violaxanthin to neoxanthin, which is then isomerized from 9-trans to 9-cis (neoxanthin). The last step of the biosynthetic pathway that occurs in the chloroplasts is the oxidative cleavage of 9-cis-neoxanthin and/or 9-cis-violaxanthin to xanthoxin, which is further catalyzed by 9-cis-epoxycarotenoid dioxygenase (NCED). NCED is known to be the key rate-limiting enzyme in the ABA biosynthesis and it is responsible for catalyzing the first non-reversible step in the pathway [15]. The formed xanthoxin then moves from the chloroplasts to the cytosol, where it is converted to abscisic aldehyde, and finally to ABA. ABA accumulation in plants occurs not only through activation of ABA biosynthesis but also by the hydrolysis of its glycosyl ester (i.e., ABA-GE) [16][17], which can be utilized to maintain the high levels of ABA in plants during drought [18][19][20].
Since the first studies linking ABA with stomatal closure under drought, a number of results have led to the critical assumption that the pool of ABA resulting in stomatal closure in leaves is mostly synthesized in the root tips [21][22]. However, this conventional view of root-to-shoot ABA signaling has recently been challenged by studies using a number of experimental approaches, including the reciprocal grafting between ABA biosynthetic mutant and wild-type plants and exogenous labeled ABA [23][24][25][26][27]. In fact, these studies clearly demonstrate that although multiple organs and tissues can accumulate small amounts of ABA, leaves are the predominant site for ABA biosynthesis, and they are even responsible to export ABA to the roots, maintaining normal root levels of ABA levels and determining root architecture and growth [24][26][27]. Inside leaves, phloem companion cells, guard cells, and mesophyll cells have been demonstrated to be capable of synthesizing ABA [28][29][30][31]. In addition, ABA biosynthesis has been recently observed to occur in parenchyma cells around the vascular tissues [32]. Regarding the ABA transport within plants, the recent identification of a number of transmembrane ABA transporters strongly suggests that the movement of this hormone is actively regulated in an intercellular network [9]. However, the fine regulation of ABA biosynthesis and transport among different plant tissues remains a matter of debate [9].
The major production of ABA in leaves, not roots, during drought seems considerably more advantageous for plants to avoid excessive declines in water potential as leaves represent the main organ exchanging (and mostly losing) water with the atmosphere. For instance, a de novo biosynthesis of ABA over an extremely short time-frame following leaf exposure to high vapor pressure deficit (VPD) has been demonstrated to allow an efficient stomatal closure in angiosperm species [33][34]. Declines in leaf water potential to a threshold leaf water potential results in major increases in foliar ABA [35][36] and such threshold water potential strongly coincides with bulk leaf turgor loss point [14][34][37][38]. Although the water potential at turgor loss is broadly accepted as the threshold for the major increment in foliar ABA biosynthesis, it is still uncertain whether changes in turgor itself or changes in cell volume are the main signal up-regulating the enzymes responsible for ABA production [14][39]. In addition, given the lower water potential in the guard cells and the mesophyll cells nearby stomata in transpiring leaves [40], small levels of ABA are expected to be produced in these cells as transpirational demand increases, resulting in stomatal closure even prior to leaf turgor loss point [34].
3. Regulation of ABA Levels and Perception in Crop Species
Ongoing climate change coupled with the projected increase in human population by over 2.4 billion until 2050 [41][42] represents a major challenge for the agricultural sector [43]. Over the next decades, climate change is expected to result in considerable rise in the intensity and frequency of drought events. In this water-limited scenario, achieving ‘more crop per drop’ (i.e., crop water productivity that is defined as the mass of agricultural produce per unit of water consumed) is a critical target for food production. Especially when considering that 70% of the available fresh water is used for crop production and that drought is expected to cause the largest yield reductions relative to any other abiotic and biotic stresses [44]. Therefore, enhancing crop WUE is still foreseen as a good means for saving water in agriculture [45] and has proven to be an accurate target in both genotype selection [46][47][48] and engineering [49].
The WUE can be determined both at the plant and leaf levels. At the leaf level, the photosynthesis-to-stomatal conductance ratio is defined as the intrinsic water-use efficiency (WUEi). This parameter is typically determined in studies aimed at improving crop yield while saving soil water and mitigating yield limitations by water deficit [49][50]. In this context, the importance of ABA for WUEi improvement is well-known due to its central role in regulating plant-water relations, specially by modulating stomatal conductance in several crop species [51][52][53][54]. As outlined above, the direct influence of ABA on WUEi in angiosperms has already been demonstrated and several studies have concluded that plant responses driven by ABA depend on the severity and the duration of the drought events [55]. Particularly under short-term water deficit, ABA induces stomatal closure through its direct action on guard cells and modulates mesophyll conductance without restraining CO2 fixation [2][6][32]. During severe and prolonged drought conditions, ABA can trigger further alterations at the transcriptome level, including genes encoding for LEA (late embryogenesis abundant) proteins [56]. Besides, it can alter the levels of osmoproctectans [57] and cause morphological changes in stomatal size and density [58], leaf size, and shoot and root development [1][59]. All these alterations strongly affect plant water balance and WUE in the long-term [60]. In addition, ABA have been observed to regulate WUE through a tissue-specific action on hydraulic conductivity. Particularly in leaves, ABA has been found to decrease the hydraulic conductance by reducing the permeability of bundle sheath aquaporins [2][3][61] and also indirectly controlling mesophyll hydraulic conductances [5][62]. In the roots, ABA has been observed to increase hydraulic conductivity, facilitating water uptake under non-transpiring conditions [1][63]. Therefore, ABA signaling network can be modulated to reduce transpiration and increase crop water productivity under stressful conditions [55][64].
The ABA signal transduction pathway has recently been well documented, consisting of pyrabactin resistance/pyrabactin resistance-like/regulatory component of ABA receptors (PYR/PYL/RCAR), clade A type 2C protein phosphatases (PP2Cs), and SNFl related protein kinase 2 (SnRK2s) [65][66]. The first attempts to use ABA in large scale agriculture were constrained by the chemical instability of ABA (e.g., under UV-light), its relatively expensive production, and its rapid cellular catabolism [67]. The modulation of ABA signaling, however, can be efficiently achieved through genetically engineered plants with an overexpression of ABA receptors [68][69][49][70] and signal transduction components [71][72], as well as through chemical intervention with ABA agonists [73][74][75][76][77].
Over the last years, studies on ABA overexpression receptors have led to promising results both in the field [78][79] and under controlled conditions [49][70]. The overexpression of PYL/RCAR receptors induces a higher plant sensitivity to ABA, leading to a globally enhanced WUE and drought resistance, as previously demonstrated in rice, poplar, and wheat [69][70][80][81]. The actual challenge, however, is to balance the improvements in WUE against the negative effects on growth due to ABA oversensitivity. Recent studies on plants overexpressing PYL receptors have shown an opposite effect on growth and stress adaptation and this may be due to the specific receptor choice. Indeed, the overexpression of some specific subfamilies result in plants with less transpiration for a similar leaf area and biomass compared to the wild-types, thus leading to an increased WUE [64]. In particular, PYL12/RCAR6 and PYL4/RCAR10 (subfamily I and II) overexpressing lines in Arabidopsis possessed a balance of reduced water use with negligible effects on growth and a “water-saving” phenotype that resulted in a significant improvement in total biomass relative to water use gains [49]. Similar observations have been made in transgenic wheat and in rice, in which the overexpression of specific PYL receptors has led to a reduced transpiration and a concomitant increase in photosynthetic activity compared to the wild-type, improving grain production per liter of water and protecting productivity during water deficit [69][70][79].
A faster and a more manageable alternative to the expression of genes for increasing crop’s WUE and conferring drought resistance is the use of ABA agonists [75][82], which are small synthetic molecules binding ABA receptors and activating ABA signaling pathway [83]. Among the ABA agonists, the first synthetized molecule Pyrabactin has been identified in a chemical genetic screen for seed germination inhibitors and it has been instrumental in showing how ABA binds the PYR/PYL receptor family [66]. The direct application of this molecule is not practical for agricultural purposes since its major effects are in seeds rather than in vegetative tissues. However, the examination of the structure of Pyrobactin and its interaction with ABA receptors has provided a framework for the design of novel ABA agonists [82]. As a consequence, several ABA agonists have been developed during the last ten years, with promising results in increasing crop water productivity [84]. An example is represented by Quinabactin, which appeared to be an overall better ABA agonist than Pyrabactin, inducing ABA responses both in seeds and vegetative tissues. Application of Quinabactin in plants resulted in improved water use upon drought stress [73]. This molecule targets all of the dimeric subfamily III ABA receptors, affecting guard cell closure to prevent water loss from detached leaves and conferring drought tolerance in both Arabidopsis and soybean during drought [73]. In addition, Quinabactin has shown effect in rapeseed and tomato, inhibiting germination and eliciting drought stress responses [85][86][87]. Another example concerns a tetrafluoro derivative of Quinabactin, AMF4, which seems to be more effective than Quinabactin due to its higher stability and bioavailability, even targeting only a subset of ABA receptors [73][88]. Finally, a recent example is represented by the agonist B2, which was shown to improve drought tolerance in wheat by increasing root biomass and preventing leaf dehydration as well as amplifying antioxidant responses and enhancing the photosynthetic performances [77].
A recent innovative approach aims at combining the application of agrochemicals on genetically modified crops such as AMF4 on PYL2 overexpressed plants or mandipropamid on engineered PYR1 receptor (PYR1MANDI) [74]. The mandipropamid-PYR1MANDI system resulted in increased seedling survival during drought and transcriptional responses similar to those induced by ABA. Recently, Cao et al. [88] optimized the synthesis of new molecules based on the Quinabactin backbone and applied them to transgenic Arabidopsis and soybean plants with an abiotic stress-inducible AtPYL2 overexpression. This combined approach dramatically increased drought resistance in these plants, making this system a compelling alternative strategy to manipulate plant water use. Although the utilization of agrochemicals acting as ABA receptor agonists combined with transgenic approaches to increase ABA signaling holds great promise for the production of plants with enhanced WUE, the translation of these applications at field level into practical improvements in crop yield can be challenging [89][90]. For instance, as previously stated, a recent study conducted on tomato has highlighted a higher vulnerability to embolism of the vascular system of the transgenic line sp12 which overproduces ABA [63]. A reduced hydraulic safety margin can compromise the maintenance of hydraulic conductivity during periods of soil water deficit, with negative consequences not only for photosynthesis and productivity, but also for plant survival [90]. Given the importance of the potential use of plants with increased ABA biosynthesis and signaling to the future of agriculture in a world facing an ongoing climate change, it is of critical importance to confirm whether increased levels of ABA result in more vulnerable xylem and to understand the underlying mechanisms linking ABA signaling and xylem functioning. Important questions remain unanswered, especially those related to the extent of variation in embolism resistance among single crop species and their different varieties [91][92], including the relationship with genotypic variations in endogenous ABA content. Therefore, to optimize WUE strategies in the field, a complete understanding of the changes at the hydraulic and metabolic levels induced by the manipulation of ABA levels is needed. Timely investments in research at different levels will likely allow to improve crop resilience and yields in a water-limited scenario.
This entry is adapted from the peer-reviewed paper 10.3390/app10186322
References
- Rosales, M.; Maurel, C.; Nacry, P. Abscisic acid coordinates dose-dependent developmental and hydraulic responses of roots to water deficit. Plant Physiol. 2019, 180, 2198–2211.
- Pantin, F.; Monnet, F.; Jannaud, D.; Costa, J.M.; Renaud, J.; Muller, B.; Simonneau, T.; Genty, B. The dual effect of abscisic acid on stomata. New Phytol. 2013, 197, 65–72.
- Coupel-Ledru, A.; Tyerman, S.D.; Masclef, D.; Lebon, E.; Christophe, A.; Edwards, E.J.; Simonneau, T. Abscisic acid down-regulates hydraulic conductance of grapevine leaves in isohydric genotypes only. Plant Physiol. 2017, 175, 1121–1134.
- Mizokami, Y.; Noguchi, K.; Kojima, M.; Sakakibara, M.; Terashima, I. Effects of instantaneous and growth CO2 levels and abscisic acid on stomatal and mesophyll conductances. Plant Cell Environ. 2018, 42, 1257–1269.
- Sade, N.; Galle, A.; Flexas, J.; Lerner, S.; Peleg, G.; Yaaran, A.; Moshelion, M. Differential tissue-specific expression of NtAQP1 in Arabidopsis thaliana reveals a role for this protein in stomatal and mesophyll conductance of CO2 under standard and salt-stress conditions. Planta 2014, 239, 357–366.
- Sorrentino, G.; Haworth, M.; Said Wahbi, T.M.; Zuomin, S.; Centritto, M. Abscisic acid induces rapid reductions in mesophyll conductance to carbon dioxide. PLoS ONE 2016, 11, e0148554.
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929.
- Wang, Z.-Y.; Xiong, L.; Li, W.; Zhu, J.-K.; Zhu, J. The plant cuticle is required for osmotic stress regulation of abscisic acid biosynthesis and osmotic stress tolerance in Arabidopsis. Plant Cell 2011, 23, 1971–1984.
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522.
- Nambara, E.; Marion-Poll, A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185.
- Taylor, I.B.; Sonneveld, T.; Bugg, T.D.H.; Thompson, A.J. Regulation and manipulation of the biosynthesis of abscisic acid, including the supply of xanthophyll precursors. J. Plant Growth Regul. 2005, 24, 253–273.
- Neuman, H.; Galpaz, N.; Cunningham, F.X.J.; Zamir, D.; Hirschberg, J. The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J. 2014, 78, 80–93.
- McAdam, S.A.M.; Sussmilch, F.C.; Brodribb, T.J.; Ross, J.J. Molecular characterization of a mutation affecting abscisic acid biosynthesis and consequently stomatal responses to humidity in an agriculturally important species. AoB Plants 2015, 27, 7.
- Sussmilch, F.C.; Brodribb, T.J.; McAdam, S.A.M. Up-regulation of NCED3 and ABA biosynthesis occur within minutes of a decrease in leaf turgor but AHK1 is not required. J. Exp. Bot. 2017, 68, 2913–2918.
- Thompson, A.J.; Jackson, A.C.; Symonds, R.C.; Mulholland, B.J.; Dadswell, A.R.; Blake, P.S.; Burbidge, A.; Taylor, I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes overproduction of abscisic acid. Plant J. 2000, 23, 363–374.
- Dietz, K.J.; Sauter, A.; Wichert, K.; Messdaghi, D.; Hartung, W. Extracellular β-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J. Exp. Bot. 2000, 51, 937–944.
- Seiler, C.; Harshavardhan, V.T.; Rajesh, K.; Reddy, P.S.; Strickert, M.; Rolletschek, H.; Scholz, U.; Wobus, U.; Sreenivasulu, N. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J. Exp. Bot. 2011, 62, 2615–2632.
- Lee, K.H.; Piao, H.L.; Kim, H.-Y.; Choi, S.M.; Jiang, F.; Hartung, W.; Hwang, I.; Kwak, J.M.; Lee, I.-J.; Hwang, I. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 2006, 126, 1109–1120.
- Xu, M.; Zhang, B.; Su, X.; Zhang, S.; Huang, M. Reference gene selection for quantitative real-time polymerase chain reaction in Populus. Anal. Biochem. 2011, 408, 337–339.
- Han, Y.; Watanabe, S.; Shimada, H.; Sakamoto, A. Dynamics of the leaf endoplasmic reticulum modulate β-glucosidase-mediated stress-activated ABA production from its glucosyl ester. J. Exp. Bot. 2020, 71, 2058–2071.
- Zhang, J.; Schurr, U.; Davies, W.J. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J. Exp. Bot. 1987, 38, 1174–1181.
- Zhang, J.; Davies, W.J. Abscisic acid produced in dehydrating roots may enable the plant tomeasure the water status of the soil. Plant Cell Environ. 1989, 12, 73–81.
- Holbrook, N.M.; Shashidhar, V.R.; James, R.A.; Munns, R. Stomatal control in tomato with ABA-deficient roots: Response of grafted plants to soil drying. J. Exp. Bot. 2002, 53, 1503–1514.
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacarías, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol. 2015, 56, 2457–2466.
- McAdam, S.A.M.; Brodribb, T.J.; Ross, J.J. Shoot-derived abscisic acid promotes root growth. Plant Cell Environ. 2016, 39, 652–659.
- McAdam, S.A.M.; Manzi, M.; Ross, J.J.; Brodribb, T.J.; Gómez-Cadenas, A. Uprooting an abscisic acid paradigm: Shoots are the primary source. Plant Signal. Behav. 2016, 11, 11–12.
- Zhang, F.; Sussmilch, F.; Nichols, D.S.; Cardoso, A.A.; Brodribb, T.J.; McAdam, S.A.M. Leaves, not roots or floral tissue, are the main site of rapid, external pressure-induced ABA biosynthesis in angiosperms. J. Exp. Bot. 2018, 69, 1261–1267.
- Bauer, H.; Ache, P.; Lautner, S.; Fromm, J.; Hartung, W.; Al-Rasheid, K.A.S.; Sonnewald, S.; Sonnewald, U.; Kneitz, S.; Lachmann, N.; et al. The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr. Biol. 2013, 23, 53–57.
- Kuromori, T.; Sugimoto, E.; Shinozaki, K. Intertissue signal transfer of abscisic acid from vascular cells to guard cells. Plant Physiol. 2014, 164, 587–1592.
- McAdam, S.A.M.; Brodribb, T.J. Mesophyll cells are the main site of abscisic acid. Plant Physiol. 2018, 177, 911–917.
- Merilo, E.; Yarmolinsky, D.; Jalakas, P.; Parik, H.; Tulva, I.; Rasulov, B.; Kilk, K.; Kollist, H. Stomatal VPD response: There is more to the story than ABA. Plant Physiol. 2018, 176, 851–864.
- Brunetti, C.; Gori, A.; Marino, G.; Latini, P.; Sobolev, A.P.; Nardini, A.; Haworth, M.; Giovannelli, A.; Capitani, D.; Loreto, F.; et al. Dynamic changes in ABA content in water-stressed Populus nigra: Effects on carbon fixation and soluble carbohydrates. Ann. Bot. 2019, 124, 627–643.
- McAdam, S.A.M.; Sussmilch, F.C.; Brodribb, T.J. Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ. 2016, 39, 485–491.
- Cardoso, A.A.; Brodribb, T.J.; Kane, C.N.; DaMatta, F.M.; McAdam, S.A.M. Osmotic adjustment and hormonal regulation of stomatal responses to vapour pressure deficit in sunflower. AoB Plants 2020, 12, plaa025.
- Zabadal, T.J. A water potential threshold for the increase of abscisic acid in leaves. Plant Physiol. 1974, 53, 125–127.
- Beardsell, M.F.; Cohen, D. Relationships between leaf water status, abscisic acid levels, and stomatal resistance in maize and sorghum. Plant Physiol. 1975, 56, 207–212.
- Pierce, M.; Raschke, K. Synthesis and metabolism of abscisic acid in detached leaves of Phaseolus vulgaris L. after loss and recovery of turgor. Planta 1981, 153, 156–165.
- McAdam, S.A.M.; Brodribb, T.J. Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapor pressure deficit across land plants. Plant Physiol. 2016, 171, 2008–2016.
- Sack, L.; John, G.P.; Buckley, T.N. ABA accumulation in dehydrating leaves is associated with decline in cell volume not turgor pressure. Plant Physiol. 2018, 176, 489–495.
- Buckley, T.N.; John, G.P.; Scoffoni, C.; Sack, L. How does leaf anatomy influence water transport outside the xylem? Plant Physiol. 2015, 168, 1616–1635.
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818.
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295.
- Sunderland, T.C.; Rowland, D. Forests, land use, and challenges to climate stability and food security. In Sustainable Food and Agriculture; Campanhola, C., Pandey, S., Eds.; The Food and Agriculture Organization of the United Nations and Academic Press: Cambridge, MA, USA, 2019; pp. 95–116.
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87.
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103.
- Flexas, J.; Galmés, J.; Gallé, A.; Gulías, J.; Pou, A.; Ribas-Carbo, M.; Tomàs, M.; Medrano, H. Improving water use efficiency in grapevines: Potential physiological targets for biotechnological improvement. Aust. J. Grape Wine Res. 2010, 16, 106–121.
- Bunce, J.A. Variation among soybean cultivars in mesophyll conductance and leaf water use efficiency. Plants 2016, 5, 44.
- Li, C.; Jackson, P.; Lu, X.; Xu, C.; Cai, Q.; Basnayake, J.; Lakshmanan, P.; Ghannoum, O.; Fan, Y. Genotypic variation in transpiration efficiency due to differences in photosynthetic capacity among sugarcane-related clones. J. Exp. Bot. 2017, 68, 2377–2385.
- Yang, Z.; Liu, J.; Tischer, S.V.; Christmann, A.; Windisch, W.; Schnyder, H.; Grill, E. Leveraging abscisic acid receptors for efficient water use in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 6791–6796.
- Nadal, M.; Flexas, J. Variation in photosynthetic characteristics with growth form in a water-limited scenario: Implications for assimilation rates and water use efficiency in crops. Agric. Water Manag. 2019, 216, 457–472.
- Thompson, A.J.; Andrews, J.; Mulholland, B.J.; McKee, J.M.T.; Hilton, H.W.; Horridge, J.S.; Farquhar, G.D.; Smeeton, R.C.; Smillie, I.R.A.; Black, C.R.; et al. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiol. 2007, 143, 1905–1917.
- Tardieu, F.; Zhang, J.; Katerji, N.; Bethenod, O.; Palmer, S.; Davies, W.J. Xylem ABA controls the stomatal conductance of field-grown maize subjected to soil compaction or soil drying. Plant Cell Environ. 1992, 15, 193–197.
- Saradadevi, R.; Bramley, H.; Siddique, K.H.; Edwards, E.; Palta, J.A. Contrasting stomatal regulation and leaf ABA concentrations in wheat genotypes when split root systems were exposed to terminal drought. Field Crop. Res. 2014, 165, 5–14.
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571.
- Negin, B.; Moshelion, M. The evolution of the role of ABA in the regulation of water-use efficiency: From biochemical mechanisms to stomatal conductance. Plant Sci. 2016, 251, 82–89.
- Todaka, D.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. ABA-responsive gene expression in response to drought stress: Cellular regulation and long-distance signaling. Adv. Bot. Res. 2019, 92, 83–113.
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of leaf starch degradation by abscisic acid is important for osmotic stress tolerance in plants. Plant Cell 2016, 28, 1860–1878.
- Chater, C.C.C.; Oliver, J.; Casson, S.; Gray, J.E. Putting the brakes on: Abscisic acid as a central environmental regulator of stomatal development. New Phytol. 2014, 202, 376–391.
- Arend, M.; Schnitzler, J.-P.; Ehlting, B.; Hänsch, R.; Lange, T.; Rennenberg, H.; Himmelbach, A.; Grill, E.; Fromm, J. Expression of the Arabidopsis mutant ABI1 gene alters abscisic acid sensitivity: Stomatal development, and growth morphology in gray poplars. Plant Physiol. 2009, 151, 2110–2119.
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679.
- Prado, K.; Maurel, C. Regulation of leaf hydraulics: From molecular to whole plant levels. Front. Plant Sci. 2013, 4, 255.
- Shatil-Cohen, A.; Attia, Z.; Moshelion, M. Bundle-sheath cell regulation of xylem-mesophyll water transport via aquaporins under drought stress: A target of xylem-borne ABA? Plant J. 2011, 67, 72–80.
- Lamarque, L.; Delzon, S.; Toups, H.; Gravel, A.-I.; Corso, D.; Badel, E.; Burllet, R.; Charrier, G.; Cochard, H.; Jansen, S.; et al. Over-accumulation of abscisic acid in transgenic tomato plants increases the risk of hydraulic failure. Plant Cell Environ. 2020, 43, 548–562.
- Dejonghe, W.; Cutler, S.R. Abscisic acid as a gateway for the crops of tomorrow. Adv. Bot. Res. 2019, 92, 341–370.
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 2009, 324, 1064–1068.
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic acid inhibits Type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071.
- Yang, Z.; Liu, J.; Poree, F.; Schaeufele, R.; Helmke, H.; Frackenpohl, J.; Lehr, S.; von Koskull-Döring, P.; Christmann, A.; Schnyder, H.; et al. Abscisic acid receptors and coreceptors modulate plant water use efficiency and water productivity. Plant Physiol. 2019, 180, 1066–1080.
- Zhao, Y.; Chan, Z.; Gao, J.; Xing, L.; Cao, M.; Yu, C.; Hu, Y.; You, J.; Shi, H.; Zhu, Y.; et al. ABA receptor PYL9 promotes drought resistance and leaf senescence. Proc. Natl. Acad. Sci. USA 2016, 113, 1949–1954.
- Mega, R.; Abe, F.; Kim, J.S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J.; et al. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159.
- Mega, R.; Tsujimoto, H.; Okamoto, M. Genetic manipulation of abscisic acid receptors enables modulation of water use efficiency. Plant Signal. Behav. 2019, 14, e1642039.
- Oneto, C.D.; Otegui, M.E.; Baroli, I.; Beznec, A.; Faccio, P.; Bossio, E.; Blumwald, E.; Lewi, D. Water deficit stress tolerance in maize conferred by expression of an isopentenyltransferase (IPT) gene driven by a stress- and maturation-induced promoter. J. Biotechnol. 2016, 220, 66–77.
- Kudo, M.; Kidokoro, S.; Yoshida, T.; Mizoi, J.; Kojima, M.; Takebayashi, T.; Sakakibara, H.; Fernie, A.R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. A gene-stacking approach to overcome the trade-off between drought stress tolerance and growth in Arabidopsis. Plant J. 2019, 97, 240–256.
- Okamoto, M.; Peterson, F.C.; Defries, A.; Park, S.Y.; Endo, A.; Nambara, E.; Volkman, B.F.; Cutler, S.R. Activation of dimeric ABA receptors elicits guard cell closure, ABA-regulated gene expression, and drought tolerance. Proc. Natl. Acad. Sci. USA 2013, 110, 12132–12137.
- Park, S.Y.; Peterson, F.C.; Mosquna, A.; Yao, J.; Volkman, B.F.; Cutler, S.R. Agrochemical control of plant water use using engineered abscisic acid receptors. Nature 2015, 520, 545–548.
- Cao, M.; Liu, X.; Zhang, Y.; Xue, X.; Zhou, X.E.; Melcher, K.; Gao, P.; Wang, F.; Zeng, L.; Zhao, Y.; et al. An ABA-mimicking ligand that reduces water loss and promotes drought resistance in plants. Cell Res. 2013, 23, 1043–1054.
- Vaidya, A.S.; Helander, J.D.; Peterson, F.C.; Elzinga, D.; Dejonghe, W.; Kaundal, A.; Park, S.Y.; Xing, Z.; Mega, R.; Takeuchi, J.; et al. Dynamic control of plant water use using designed ABA receptor agonists. Science 2019, 366, eaaw8848.
- Zhou, Y.; He, R.; Guo, Y.; Liu, K.; Huang, G.; Peng, C.; Liu, Y.; Zhang, M.; Li, Z.; Duan, L. A novel ABA functional analogue B2 enhances drought tolerance in wheat. Sci. Rep. 2019, 9, 2887.
- Chang, Y.; Nguyen, B.H.; Xie, Y.; Xiao, B.; Tang, N.; Zhu, W.; Mou, T.; Xiong, L. Co-overexpression of the constitutively active form of OsbZIP46 and ABA-activated protein kinase SAPK6 improves drought and temperature stress resistance in rice. Front. Plant Sci. 2017, 8, 1102.
- Verma, R.K.; Santosh Kumar, V.V.; Yadav, S.K.; Pushkar, S.; Rao, M.V.; Chinnusamy, V. Overexpression of ABA Receptor PYL10 gene confers drought and cold tolerance to indica rice. Front. Plant Sci. 2019, 10, 1488.
- Kim, D.H. Mechanism of ABA signal transduction: Agricultural highlights for improving drought tolerance. J. Plant Biol. 2014, 57, 1–8.
- Yu, J.; Ge, H.; Wang, X.; Tang, R.; Wang, Y.; Zhao, F.; Lan, W.; Luan, S.; Yang, L. Overexpression of pyrabactin resistance-like abscisic acid receptors enhances drought, osmotic, and cold tolerance in transgenic poplars. Front. Plant Sci. 2017, 8, 1752.
- Hao, Q.; Yin, P.; Yan, C.; Yuan, X.; Li, W.; Zhang, Z.; Liu, L.; Wang, J.; Yan, N. Functional mechanism of the abscisic acid agonist pyrabactin. J. Biol. Chem. 2010, 285, 28946–28952.
- Rodriguez, P.L.; Lozano-Juste, J. Unnatural agrochemical ligands for engineered abscisic acid receptors. Trends Plant Sci. 2015, 20, 330–332.
- Helander, J.D.M.; Vaidya, A.S.; Cutler, S.R. Chemical manipulation of plant water use. Bioorg. Med. Chem. 2016, 24, 493–500.
- Naeem, M.S.; Dai, L.; Ahmad, F.; Ahmad, A.; Li, J.; Zhang, C. AM1 is a potential ABA substitute for drought tolerance as revealed by physiological and ultra-structural responses of oilseed rape. Acta Physiol. Plant. 2016, 38, 183.
- Xiong, J.L.; Dai, L.L.; Ma, N.; Zhang, C.L. Transcriptome and physiological analyses reveal that AM1 as an ABA-mimicking ligand improves drought resistance in Brassica napus. Plant Growth Regul. 2018, 85, 73–90.
- González-Guzmán, M.; Rodríguez, L.; Lorenzo-Orts, L.; Pons, C.; Sarrión-Perdigones, A.; Fernández, M.A.; Peirats-Llobet, M.; Forment, J.; Moreno-Alvero, M.; Cutler, S.R.; et al. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J. Exp. Bot. 2014, 65, 4451–4464.
- Cao, M.J.; Zhang, Y.L.; Liu, X.; Huang, H.; Zhou, X.E.; Wang, W.L.; Zeng, A.; Zhao, C.-Z.; Si, T.; Du, J.; et al. Combining chemical and genetic approaches to increase drought resistance in plants. Nat. Commun. 2017, 8, 1183.
- Blum, A. Towards a conceptual ABA ideotype in plant breeding for water limited environments. Funct. Plant Biol. 2015, 42, 502–513.
- Nuccio, M.L.; Paul, M.; Bate, N.J.; Cohn, J.; Cutler, S.R. Where are the drought tolerant crops? An assessment of more than two decades of plant biotechnology effort in crop improvement. Plant Sci. 2018, 273, 110–119.
- Ahmad, H.B.; Lens, F.; Capdeville, G.; Burlett, R.; Lamarque, L.J.; Delzon, S. Intraspecific variation in embolism resistance and stem anatomy across four sunflowers. Physiol. Plant. 2017, 163, 59–72.
- Neufeld, H.S.; Grantz, D.A.; Meinzer, F.C.; Goldstein, G.; Crisosto, G.M.; Crisosto, C. Genotypic variability in vulnerability of leaf xylem to cavitation in water-stressed and well-irrigated sugarcane. Plant Physiol. 1992, 100, 1020–1028.
