As the scale of the livestock industry has grown with the increase in the demand for livestock and poultry products, gaseous emissions, an unwanted side effect of livestock and poultry production, are also increasing. Various mitigation technologies have been developed to reduce such air pollution, and the mitigation technologies are divided mainly into “source-based type” (meant to fundamentally reduce the emissions) and “end-of-pipe type” (physicochemical and biological treatment of the output from barns to reduce the release into the environment). Ultraviolet light (UV) can be considered as both end-of-pipe (treating exhaust air from barns) and source-based type (treating air inside the barn).
- environmental catalysis
- ultraviolet light
- air purification
- air pollution control
1. Introduction
2. Mechanism of UV-A Photocatalysis
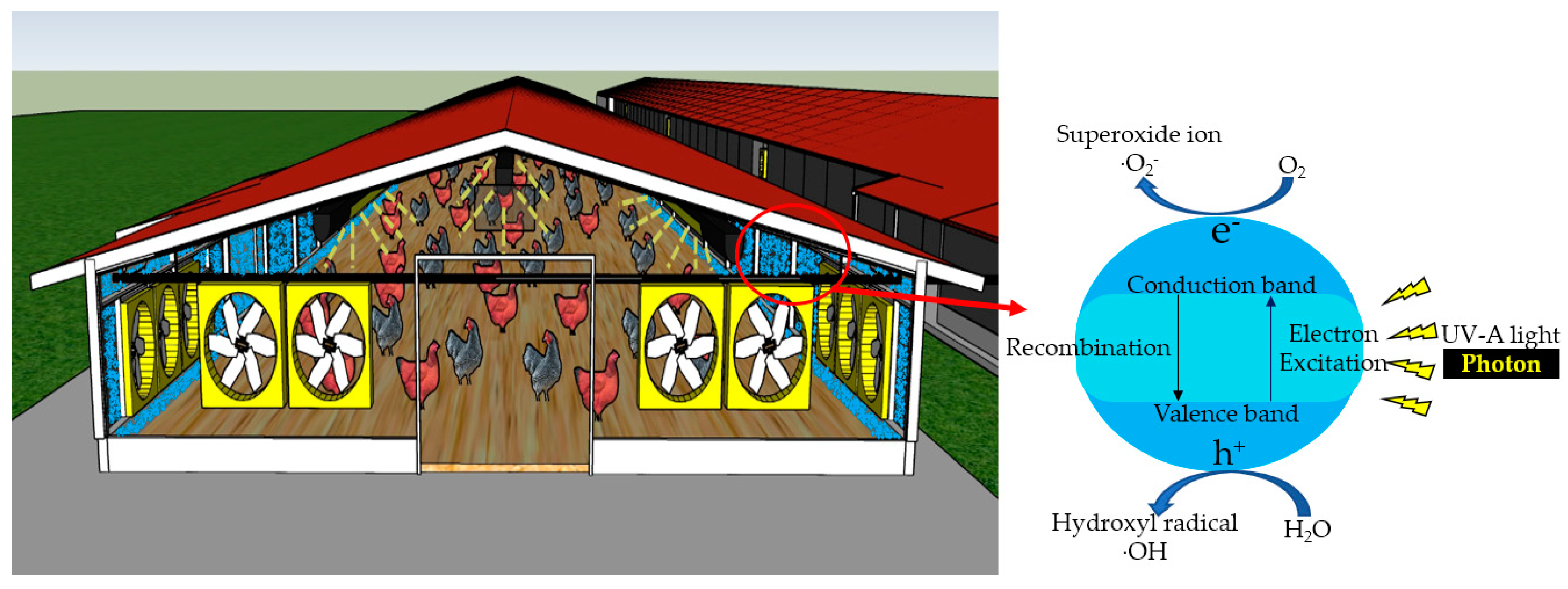
3. UV-A Photocatalysis Technology’s Effectiveness in Mitigating Targeted Air Pollutants in Livestock and Poultry Barns
3.1. Mitigation of NH3 and H2S
| Reference | Experimental Conditions |
UV-A Type (Major Wavelength) |
UV Dose (Light Intensity) |
Catalyst (Dose) |
Gas Mitigation (Mitigation %) |
|---|---|---|---|---|---|
| [28] | Lab scale Temp: 24 °C RH: 50% |
Fluorescent (365 nm) |
Not reported (0.46 mW·cm−2) |
TiO2 (approx. 1 mg·cm−2) |
NH3 (35) |
| [20] | Lab scale (simulated poultry farm) Temp: 25 ± 3 °C RH: 12% |
Fluorescent (365 nm) |
<88 mJ·cm−2 (<0.44 mW·cm−2) |
TiO2 (10 μg·cm−2) |
NH3 (9.4) H2S (N/S) |
| LED (365 nm) |
<0.97 J·cm−2 (<4.85 mW·cm−2) |
NH3 (19) H2S (N/S) |
|||
| [27] | Pilot scale (layer poultry farm) Temp: 28 ± 3 °C RH: 56% |
Fluorescent (365 nm) |
<75 mJ·cm−2 (<0.44 mW·cm−2) |
TiO2 (10 μg·cm−2) |
NH3 (5.2) |
| LED (365 nm) |
<0.82 J·cm−2 (<4.85 mW·cm−2) |
NH3 (8.7) | |||
| [26] | Pilot scale (simulated swine farm) Temp: 11 ± 3 °C RH: 34 ± 6% |
LED (367 nm) |
3.9 and 5.8 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
NH3 (9 and 11) |
| [24] | Pilot scale (simulated swine farm) Temp: 19 ± 2 °C RH: 45 ± 4% |
LED (367 nm) |
5.8 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
NH3 (6.1) |
| [22] | Swine farm (farrowing rooms) Temp: 24 °C (19–27) RH: 54% |
Not reported (315–400 nm) |
Not reported | TiO2 (7 mg·cm−2) |
NH3 (31) |
| [29] | Lab scale (simulated livestock farm) Temp: 20 ± 1 °C RH: 51% |
Not reported (368 nm) |
0.6 and 1.3 mJ·cm−2 (2.3–5.6 mW·cm−2) |
TiO2 (1.5 m2·g−1) |
H2S (4.2 and 14) |
| [25] | Swine farm (finishing rooms) Temp: 29 ± 2 °C RH: 66 ± 4% |
LED (367 nm) |
5.3 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
NH3 (N/S) H2S (26~40) |
3.2. Mitigation of VOCs and Odor
| Reference | Experimental Conditions |
UV-A Type (Major Wavelength) |
UV Dose (Light Intensity) |
Catalyst (Dose) |
VOC Mitigation (Mitigation %) |
|---|---|---|---|---|---|
| [29] | Lab scale (simulated livestock farm) Temp: 20 ± 1 °C RH: 51% |
Not reported (368 nm) |
0.6 and 1.3 mJ·cm−2 (2.3–5.6 mW·cm−2) |
TiO2 (1.5 m2·g−1) |
MT (80–87) DMS (92–96) DMDS (83–91) Butan-1-ol (93–95) AA (81–89) PA (97–98) BA (98–99) VA (98–99) |
| [30] | Lab scale (simulated livestock farm) Temp: 40 °C R: 40% |
Fluorescent (365 nm) |
12 mJ·cm−2 (0.06 mW·cm−2) |
TiO2 (10 μg·cm−2) |
DMDS (40) DEDS (81) DMTS (76) BA (87) Guaiacol (100) p-Cresol (94) |
| [19] | Pilot scale (swine finishing room) Temp: 22~26 °C RH: 36~80% |
Fluorescent (365 nm) |
<1.88 mJ·cm−2 (<0.04 mW·cm−2) |
TiO2 (10 μg·cm−2) |
p-Cresol (22) Odor (16) |
| [27] | Pilot scale (layer poultry farm) Temp: 28 ± 3 °C RH: 56% |
LED (365 nm) |
<0.82 J·cm−2 (<4.85 mW·cm−2) |
TiO2 (10 μg·cm−2) |
DEDS (47) BA (62) p-Cresol (49) Skatole (35) Odor (18) |
| [26] | Pilot scale (simulated swine farm) Temp: 11 ± 3 °C RH: 34 ± 6% |
LED (367 nm) |
2.5 and 5.8 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
Butan-1-ol (19 and 41) |
| [24] | Pilot scale (simulated swine farm) Temp: 19 ± 2 °C RH: 45 ± 4% |
LED (367 nm) |
1.3 and 3.9 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
AA (N/S and 49) BA (36 and 53) p-Cresol (N/S and 67) Indole (N/S and 32) Odor (N/S and 58) |
| [25] | Swine farm (finishing rooms) Temp: 29 ± 2 °C RH: 66 ± 4% |
LED (367 nm) |
2.9 and 5.3 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
DMDS (22 and 62) IA (N/S and 44) BA (N/S and 32) p-Cresol (32 and 40) Indole (N/S and 66) Skatole (38 and 49) Odor (N/S and 40) |
3.3. Mitigation of GHGs
| Reference | Experimental Conditions |
UV-A Type (Major Wavelength) |
UV Dose (Light Intensity) |
Catalyst (Dose) |
GHGs Mitigation (Mitigation %) |
|---|---|---|---|---|---|
| [20] | Lab scale (simulated poultry farm) Temp: 25 ± 3 °C RH: 12% |
Fluorescent (365 nm) |
<88 mJ·cm−2 (<0.44 mW·cm−2) |
TiO2 (10 μg·cm−2) |
N2O (3.3) |
| LED (365 nm) |
<0.97 J·cm−2 (<4.85 mW·cm−2) |
CO2 (3.8) N2O (10) |
|||
| [19] | Pilot scale (swine finishing room) Temp: 22~26 °C RH: 36~80% |
Fluorescent (365 nm) |
<1.88 mJ·cm−2 (<0.04 mW·cm−2) |
TiO2 (10 μg·cm−2) |
CO2 (−3.1) N2O (8.7) |
| [27] | Pilot scale (layer poultry farm) Temp: 28 ± 3 °C RH: 56% |
Fluorescent (365 nm) |
<75 mJ·cm−2 (<0.44 mW·cm−2) |
TiO2 (10 μg·cm−2) |
N2O (7.5) |
| LED (365 nm) |
<0.82 J·cm−2 (<4.85 mW·cm−2) |
N2O (13) | |||
| [24] | Pilot scale (simulated swine farm) Temp: 19 ± 2 °C RH: 45 ± 4% |
LED (367 nm) |
2.5 and 3.9 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
N2O (9.0 and 4.3) CO2 (N/S and −25.8) |
| [25] | Swine farm (finishing rooms) Temp: 29 ± 2 °C RH: 66 ± 4% |
LED (367 nm) |
2.9 and 5.3 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
N2O (9.4 and 12) CO2 (−33.7 and −27.8) |
| [22] | Swine farm (farrowing rooms) Temp: 24 °C (19–27) RH: 54% |
Not reported (315–400 nm) |
Not reported | TiO2 (7 mg·cm−2) |
CH4 (15) CO2 (11) N2O (4.2) |
| [21] | Swine farm (weaning rooms) Temp: 26 °C (24~30) RH: 56% (52~90) |
Not reported (315–400 nm) |
Not reported | TiO2 (7 mg·cm−2) |
CH4 (27) |
3.4. Mitigation of Pollutants
| Reference | Experimental Conditions |
UV-A Type (Major Wavelength) |
UV Dose (Light Intensity) |
Catalyst (Dose) |
Mitigation (Mitigation %) |
|---|---|---|---|---|---|
| [20] | Lab scale (simulated poultry farm) Temp: 25 ± 3 °C RH: 12% |
Fluorescent (365 nm) |
<88 mJ·cm−2 (<0.44 mW·cm−2) |
TiO2 (10 μg·cm−2) |
O3 (24) |
| LED (365 nm) |
<0.97 J·cm−2 (<4.85 mW·cm−2) |
O3 (48) | |||
| [24] | Pilot scale (simulated swine farm) Temp: 19 ± 2 °C RH: 45 ± 4% |
LED (367 nm) |
1.3 and 5.8 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
O3 (100 and 100) |
| [27] | Pilot scale (layer poultry farm) Temp: 28 ± 3 °C RH: 56% |
Fluorescent (365 nm) |
<75 mJ·cm−2 (<0.44 mW·cm−2) |
TiO2 (10 μg·cm−2) |
O3 (100) |
| LED (365 nm) |
<0.82 J·cm−2 (<4.85 mW·cm−2) |
O3 (100) | |||
| [21] | Swine farm (weaning rooms) Temp: 26 °C (24~30) RH: 56% (52~90) |
Not reported (315–400 nm) |
Not reported | TiO2 (7 mg·cm−2) |
PM 10 (17) FCR (−12) |
| [25] | Swine farm (finishing rooms) Temp: 29 ± 2 °C RH: 66 ± 4% |
LED (367 nm) |
5.3 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
O3 (100) |
| [23] | Swine farm (finishing rooms) Temp: 29 ± 2 °C RH: 66 ± 4% |
LED (367 nm) |
5.3 mJ·cm−2 (0.41 mW·cm−2) |
TiO2 (10 μg·cm−2) |
CFU (49~51) PM (N/S) |
This entry is adapted from the peer-reviewed paper 10.3390/catal12070782
References
- Buijsman, E.; Erisman, J.-W. Wet deposition of ammonium in Europe. J. Atmos. Chem. 1988, 6, 265–280.
- Casey, J.A.; Kim, B.F.; Larsen, J.; Price, L.B.; Nachman, K.E. Industrial food animal production and community health. Curr. Environ. Health Rep. 2015, 2, 259–271.
- Herrero, M.; Gerber, P.; Vellinga, T.; Garnett, T.; Leip, A.; Opio, C.; Westhoek, H.; Thornton, P.K.; Olesen, J.; Hutchings, N. Livestock and greenhouse gas emissions: The importance of getting the numbers right. Anim. Feed. Sci. Technol. 2011, 166, 779–782.
- Rappert, S.; Müller, R. Odor compounds in waste gas emissions from agricultural operations and food industries. Waste Manag. 2005, 25, 887–907.
- Schiffman, S.S. Livestock odors: Implications for human health and well-being. J. Anim. Sci. 1998, 76, 1343–1355.
- Bolton, J.R.; Cotton, C.A. The Ultraviolet Disinfection Handbook; American Water Works Association: Denver, CO, USA, 2011.
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269.
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986.
- Haque, M.M.; Bahnemann, D.; Muneer, M. Photocatalytic degradation of organic pollutants: Mechanisms and kinetics. In Organic Pollutants Ten Years after the Stockholm Convention–Environmental and Analytical Update; IntechOpen: London, UK, 2012; p. 293.
- Vautier, M.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of dyes in water: Case study of indigo and of indigo carmine. J. Catal. 2001, 201, 46–59.
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164.
- Athanasekou, C.P.; Moustakas, N.G.; Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.; Dona-Rodriguez, J.M.; Romanos, G.E.; Falaras, P. Ceramic photocatalytic membranes for water filtration under UV and visible light. Appl. Catal. B Environ. 2015, 178, 12–19.
- Lee, H.J.; Park, Y.G.; Lee, S.H.; Park, J.H. Photocatalytic properties of TiO2 according to manufacturing method. Korean Chem. Eng. Res. 2018, 56, 156–161.
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027.
- Gomes, J.F.; Leal, I.; Bednarczyk, K.; Gmurek, M.; Stelmachowski, M.; Zaleska-Medynska, A.; Quinta-Ferreira, M.E.; Costa, R.; Quinta-Ferreira, R.M.; Martins, R.C. Detoxification of parabens using UV-A enhanced by noble metals—TiO2 supported catalysts. J. Environ. Chem. Eng. 2017, 5, 3065–3074.
- Jia, J.; Li, D.; Wan, J.; Yu, X. Characterization and mechanism analysis of graphite/C-doped TiO2 composite for enhanced photocatalytic performance. J. Ind. Eng. Chem. 2016, 33, 162–169.
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 179–209.
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661.
- Maurer, D.L.; Koziel, J.A. On-farm pilot-scale testing of black ultraviolet light and photocatalytic coating for mitigation of odor, odorous VOCs, and greenhouse gases. Chemosphere 2019, 221, 778–784.
- Lee, M.; Wi, J.; Koziel, J.A.; Ahn, H.; Li, P.; Chen, B.; Meiirkhanuly, Z.; Banik, C.; Jenks, W. Effects of UV-A Light Treatment on Ammonia, hydrogen sulfide, greenhouse gases, and ozone in simulated poultry barn conditions. Atmosphere 2020, 11, 283.
- Costa, A.; Chiarello, G.L.; Selli, E.; Guarino, M. Effects of TiO2 based photocatalytic paint on concentrations and emissions of pollutants and on animal performance in a swine weaning unit. J. Environ. Manag. 2012, 96, 86–90.
- Guarino, M.; Costa, A.; Porro, M. Photocatalytic TiO2 coating—To reduce ammonia and greenhouse gases concentration and emission from animal husbandries. Bioresour. Technol. 2008, 99, 2650–2658.
- Lee, M.; Koziel, J.A.; Macedo, N.R.; Li, P.; Chen, B.; Jenks, W.S.; Zimmerman, J.; Paris, R.V. Mitigation of particulate matter and airborne pathogens in swine barn emissions with filtration and UV-A photocatalysis. Catalysts 2021, 11, 1302.
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Chen, B.; Li, P.; Banik, C. Evaluation of TiO2 based photocatalytic treatment of odor and gaseous emissions from swine manure with UV-A and UV-C. Animals 2021, 11, 1289.
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Chen, B.; Li, P.; Banik, C. Mitigation of odor and gaseous emissions from swine barn with UV-A and UV-C photocatalysis. Atmosphere 2021, 12, 585.
- Lee, M.; Koziel, J.A.; Murphy, W.; Jenks, W.S.; Fonken, B.; Storjohann, R.; Chen, B.; Li, P.; Banik, C.; Wahe, L. Design and testing of mobile laboratory for mitigation of gaseous emissions from livestock agriculture with photocatalysis. Int. J. Environ. Res. Public Health 2021, 18, 1523.
- Lee, M.; Li, P.; Koziel, J.A.; Ahn, H.; Wi, J.; Chen, B.; Meiirkhanuly, Z.; Banik, C.; Jenks, W.S. Pilot-scale testing of UV-A light treatment for mitigation of NH3, H2S, GHGs, VOCs, odor, and O3 inside the poultry barn. Front. Chem. 2020, 8, 613.
- Wu, H.; Ma, J.; Li, Y.; Zhang, C.; He, H. Photocatalytic oxidation of gaseous ammonia over fluorinated TiO2 with exposed (0 0 1) facets. Appl. Catal. B Environ. 2014, 152, 82–87.
- Yao, H.; Feilberg, A. Characterisation of photocatalytic degradation of odorous compounds associated with livestock facilities by means of PTR-MS. Chem. Eng. J. 2015, 277, 341–351.
- Zhu, W.; Koziel, J.A.; Maurer, D.L. Mitigation of livestock odors using black light and a new titanium dioxide-based catalyst: Proof-of-concept. Atmosphere 2017, 8, 103.
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758.
- Alonso-Tellez, A.; Robert, D.; Keller, N.; Keller, V. A parametric study of the UV-A photocatalytic oxidation of H2S over TiO2. Appl. Catal. B Environ. 2012, 115, 209–218.
- Portela, R.; Canela, M.C.; Sánchez, B.; Marques, F.C.; Stumbo, A.M.; Tessinari, R.F.; Coronado, J.M.; Suárez, S. H2S photodegradation by TiO2/M-MCM-41 (M= Cr or Ce): Deactivation and by-product generation under UV-A and visible light. Appl. Catal. B Environ. 2008, 84, 643–650.
- Brancher, M.; Franco, D.; de Melo Lisboa, H. Photocatalytic oxidation of H2S in the gas phase over TiO2-coated glass fiber filter. Environ. Technol. 2016, 37, 2852–2864.
- Yuliati, L.; Yoshida, H. Photocatalytic conversion of methane. Chem. Soc. Rev. 2008, 37, 1592–1602.
- Cybula, A.; Klein, M.; Zaleska, A. Methane formation over TiO2-based photocatalysts: Reaction pathways. Appl. Catal. B Environ. 2015, 164, 433–442.
- Ohtani, B.; Zhang, S.-W.; Nishimoto, S.-I.; Kagiya, T. Catalytic and photocatalytic decomposition of ozone at room temperature over titanium (IV) oxide. J. Chem. Soc. Faraday Trans. 1992, 88, 1049–1053.
- Černigoj, U.; Štangar, U.L.; Trebše, P. Degradation of neonicotinoid insecticides by different advanced oxidation processes and studying the effect of ozone on TiO2 photocatalysis. Appl. Catal. B Environ. 2007, 75, 229–238.
- Hernández-Alonso, M.a.D.; Coronado, J.M.; Maira, A.J.; Soria, J.; Loddo, V.; Augugliaro, V. Ozone enhanced activity of aqueous titanium dioxide suspensions for photocatalytic oxidation of free cyanide ions. Appl. Catal. B Environ. 2002, 39, 257–267.
- Pichat, P.; Cermenati, L.; Albini, A.; Mas, D.; Delprat, H.; Guillard, C. Degradation processes of organic compounds over UV-irradiated TiO2. Effect of ozone. Res. Chem. Intermed. 2000, 26, 161–170.
- Pichat, P.; Disdier, J.; Hoang-Van, C.; Mas, D.; Goutailler, G.; Gaysse, C. Purification/deodorization of indoor air and gaseous effluents by TiO2 photocatalysis. Catal. Today 2000, 63, 363–369.
- Rodrigues-Silva, C.; Miranda, S.M.; Lopes, F.V.; Silva, M.; Dezotti, M.; Silva, A.M.; Faria, J.L.; Boaventura, R.A.; Vilar, V.J.; Pinto, E. Bacteria and fungi inactivation by photocatalysis under UVA irradiation: Liquid and gas phase. Environ. Sci. Pollut. Res. 2017, 24, 6372–6381.
