1. Historical Background
1.1. The Beginning
In 1989, Dr Yoshinari completed his Postdoctoral research fellowship at Tufts School of Medicine, Boston, USA, sponsored by Dr Allen C. Steere. At that time, there was still little knowledge about Lyme Disease, and its existence was unrecognized in many countries, including Brazil. In this respect, the ticks of the
Ixodes ricinus complex had not yet been identified in Brazil
[1]. Dr Steere suggested finding possible cases of Lyme Disease (LD) in the country. To kick-start the project, Dr Steere kindly offered basic research supplies, including the reagents necessary to perform immunoenzymatic assay (ELISA) and Western blotting to detect antibodies against
Borrelia burgdorferi. In addition, he provided a culture medium (BSK II) and a culture of the
B. burgdorferi G39/40 strain, initially isolated from
Ixodes scapularis ticks and usually employed to perform serologic tests at Tufts School of Medicine. Positive IgM and IgG control sera of North American LD patients were also donated in order to begin the research in Brazil.
1.2. The Research Schedule in Brazil
The Project was approved by the Ethics Committee of the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP) and obtained finantial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). The laboratory and outpatient dispensary were set up in 1989 at HCFMUSP in order to admit patients with suspected Borreliosis. The publication of a scientific paper on LD
[1] helped in raising awareness on the matter, and lectures were provided to educate physicians from different departments working at the institution.
The former group was composed by Prof. Natalino Hajime Yoshinari (clinical rheumatologist from Faculdade de Medicina of USP), Prof. Domingos Baggio (entomologist from Instituto de Biociências of USP) and Prof. Paulo Yasuda (microbiologist from Instituto de Biociências of USP).
1.3. The Invitation from the Brazilian Ministry of Health
In 1990, one year after setting up the laboratory at the Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo (HCFMUSP) to research LD in Brazil, the group received an invitation from the National Center for Epidemiology within the Brazilian Ministry of Health to attend to patients with suspected LD coming from all over the country (Reference Center for Study of Lyme disease in Brazil). In this respect, samples of blood, cerebrospinal fluid or ticks were forwarded to the HCFMUSP, accompanied by the patients’ clinical and epidemiological data.
A brief review of LD and general recommendations on how to deliver samples to the laboratory were published in the official journals of the Brazilian Societies of Dermatology, Rheumatology, Microbiology, Tropical Medicine, Cardiology and Clinical Pathology. Additionally, all Brazilian States Health Secretaries were invited to send samples or patients to the reference unit at the HCFMUSP. During the period between 1990 and 2021, our laboratory attended to 25.128 cases referred by Brazilian physicians from all parts of the country. This enormous casuistic gave us the possibility to outline the clinical picture and behavior of serological diagnostic tests performed on cases with suspected LD.
1.4. The First Lyme Disease Cases in Brazil
In March of 1992, Dr Alexandre de Almeida, a physician at the Instituto de Infectologia Emilio Ribas, identified two brothers aged 10 and 12 who presented skin lesions compatible with erythema migrans (EM) following tick bites
[2]. They also presented with secondary annular lesions, fever, headache, myalgia and arthralgia. The boys were from São Paulo City, but at the time, their parents were in the process of building a holiday home in Cotia County, SP, a rural area located in the Atlantic Forest. Before falling ill, the children said they had been playing with a dog that died a few days later. The area is still covered in woods which are home to both wild and domestic animals.
Dr Almeida took the advice from the HCFMUSP laboratory and performed serological diagnostic tests for Borrelia burgdorferi using the G39/40 strain. The immunoenzymatic assay (ELISA) showed positive IgM results in both boys, which was then confirmed by Western blot (WB), revealing the presence in both patients of at least two IgM bands. IgG class antibodies were determined to be negative both by ELISA and WB assays. The boys were treated with doxycycline for 30 days and recovered completely. Serological assays were performed a few weeks later, which provided negative results.
1.5. First Fieldwork Survey Conducted in Cotia County, São Paulo State
The first fieldwork survey to search for reservoir animals, which carry tick transmitters of LD and possible isolation of the etiological agent of Brazilian borreliosis, was performed in Cotia County, São Paulo State, the same place where the first cases of this zoonosis were identified. This rural area is also mentioned in scientific publications; it is located in Itapevi County and is close to both cities.
The study area consisted of an apartment building located in the southwest of Itapevi County (or Cotia County), with an area of 124.58 ha, 62.07 of which are divided into lots, and 32.80 ha consist of green areas, with secondary forests at different stages of regeneration. The climate is mesothermal, with dry winters and cool summers. Small farms with domestic animals such as horses and cows infested with ticks were observed within the area surrounding the apartment building. The study was conducted from January 1995 to June 1996. Monthly trappings were carried out for five consecutive days, always on the last week of the month
[3][4][5].
Captured animals were weighed, sexed and measured. All rodents were put down for identification. Marsupials were released. Ticks were collected on animals, taxonomically classified and kept in flasks with humid paper.
Blood and internal organs from small rodents and blood from the caudal vein of marsupials were collected. Macerated ticks and animals’ blood and organs were seeded in a culture medium to allow the growth and isolation of borrelias (BSK II and other culture mediums). Part of the collected materials was preserved in liquid nitrogen or frozen for future studies.
During this period, 134 animal species were captured, of which 46.3% were Didelphimorphia (Didelphis marsupialis and Mamosops incanus), and 53.6% were Rodentia. The following nine species of Rodentia were identified: Akodon cursor, Bolomys lasiurus, Oxymicterus hispidus, Oxymicterus nasutus, Oligoryzomys nigripes, Oryzomis angouya, Rattus norvegicus, Euryzygomatomys spinus and Cavia aperea.
A total of 88 ticks were collected from the animals, most of them belonging to the Ixodidae family. Immature ticks of the Ixodes sp at larvae or nymph stages represented 47.7% of ticks. Adult ticks were identified as Ixodes didelphidis (21.6%), Ixodes loricatus (29.5%) and Amblyomma cajennense (1.2%). It is important to point out that I.didelphidis and I. loricatus do not belong to the Ixodes ricinus complex and do not bite humans. In contrast, there are cases of Brazilian borreliosis described in the work of Brazilian researchers, who presented erythema migrans following the bite of an Amblyomms cajennense tick.
Non-motile spirochete-like microorganisms were observed through dark field microscopy analysis, used to examine peripheral blood smears or to analyze samples of animal blood, organs or macerated ticks seeded in a BSK culture medium. However, isolation and culture of motile spirochetes were never achieved in the BSK II medium, even after many modifications such as the addition of different animal sera or changing its nutritional components.
These spirochete-like structures were found in 9.7% of rodents’ blood or organ culture. Only three species of rodents provided positive cultures: Akodon cursor, Bolomys lasiurus and Oxymycterus hispidus. In total, 13% of the D. marsupialis blood culture showed positivity for spirochete-like organisms, and 36.4% of adult ticks exhibited positive results.
This research conducted in Itapevi County, São Paulo State, a risk area for Brazilian borreliosis, suggested that small rodents and marsupials could be reservoirs of this zoonosis; ticks of the species I. didelphidis and I. loricatus are probably responsible for the maintenance of spirochetes among wild animals. It is important to highlight that these hard Ixodid ticks do not bite humans and cannot transmit the disease. On the contrary, Amblyomma cajennense is the most important tick vector in the country, responsible for causing Brazilian spotted fever, and also seems to be one of the vectors and transmitters of Brazilian LD. Herein, conducted in an area at risk for LD, demonstrated for the first time how difficult it is to isolate and cultivate spiral motile spirochetes from reservoirs.
Abel et al. (2000)
[6] performed the same kind of research in Cotia County, São Paulo State, and achieved similar results. Forty-four marsupials, seventy-seven rodents and one hundred sixty-one ticks were captured throughout the Atlantic Forest region. Animal blood and organs (liver and spleen) of rodents, as well as triturated ticks, were inoculated in BSK II culture medium. Twenty-one culture samples showed spirochete-like microorganisms through dark field microscopy analysis, but not the presence of the common spiral-shaped microorganisms.
An important epidemiological feature caught the attention when reviewing the clinical history of the first two boys diagnosed with LD. Since they had had contact with a dog that had died before the children became sick, it was assumed the possibility of co-infection of Borreliosis and Babesiosis. In fact, after performing an ELISA and a WB test for
Babesia bovis [7], it was observed high titers of IgM antibodies in both boys. These surprising results drove us to compare the serological response of IgM and IgG class antibodies in 59 patients with Borreliosis with that of 40 healthy people. The study confirmed the existence of co-infection between Borreliosis and Babesiosis in the country’s areas at risk
[8].
Naka et al. (2008)
[9] also found co-infection between the etiological agent of Lyme disease-like illness and Babesiosis in children from Campo Grande County, Mato Grosso do Sul. These findings are very relevant to understanding the epidemiology of Brazilian Borreliosis because Babesiosis is transmitted by ticks of the genus
Rhipicephalus. In this regard, Babesia
bovis is transmitted by
R. microplus, while
B.
canis is transmitted by ticks of the
R. sanguineus species. The occurrence of this co-infection suggested for the first time the possible implication of ticks from the
Rhipicephalus genus as vector transmitters of the Brazilian LD-like illness. Later research conducted at the HCFMUSP demonstrated that this amazing and original hypothesis was true, indicating that ticks from the genus
Rhipicephalus also participate in Borrelia transmission in the country.
1.6. Second Fieldwork in Search of Borrelia sp. in Ticks from an Urban Forest Reserve in the State of Mato Grosso do Sul, Brazil
The absence of ticks of the Ixodes ricinus complex and difficulties in isolating and cultivating B. burgdorfer within risk areas for LD intrigued researchers from the HCFMUSP. It was wondered that if another ecosystem, different from the Atlantic Forest, where the first fieldwork had been conducted, could provide new insights.
Costa et al. (1996), who worked as a rheumatologist at Universidade Federal of Mato Grosso do Sul, located in Campo Grande, Mato Grosso do Sul State, identified the first cases of meningitis associated with LD in Brazil and also identified a total of 35 patients, including patients with EM and systemic manifestations
[10][11].
In 1997, he decided to search for the etiological agent of Brazilian LD and identify the ticks and reservoir animals that could be involved in its transmission within Campo Grande County
[12]. The research took place in an urban area of 48.5 ha, surrounded by woods inhabited by wild animals, including capybaras, the biggest rodent found in the country and responsible for the transmission of Brazilian Spotted Fever. This biological reserve belongs to the Fundação Universidade Federal do Mato Grosso do Sul and is located in proximity to the region known as Pantanal, which is a huge plain area surrounded by water during the rainy season.
The animals and ticks were captured and processed the same way as in Itapevi County. This phase lasted five days during July 1997.
A total of 128 ticks of the
Amblyomma genus were collected from 5 marsupials (
Didelphis albiventris) and 17 rodents (16
Bolomys lasiurus and 1
Rattus norvegicus). Of the ticks collected, 95 (78.9%) were in larval form, and 22 (21.1%) were nymphs; the only adult was identified as
A. cajennense. Examination under dark-field microscopy revealed spiral-shaped spirochete-like structures, most of them with little motility, in nine cultures which had been left to seed in BSK-modified medium derived from spleens and livers of the rodents, blood of marsupials and macerate of
Amlyomma sp nymphs. Such structures could not be identified by Giemsa’s staining procedure. PCR amplification (a procedure to identify microorganisms of genus
Borrelia) of DNA obtained from ticks and animal cultures using primers of flagellin and 16S rRNA, as described by Barbour et al. (1996)
[13], provided negative results.
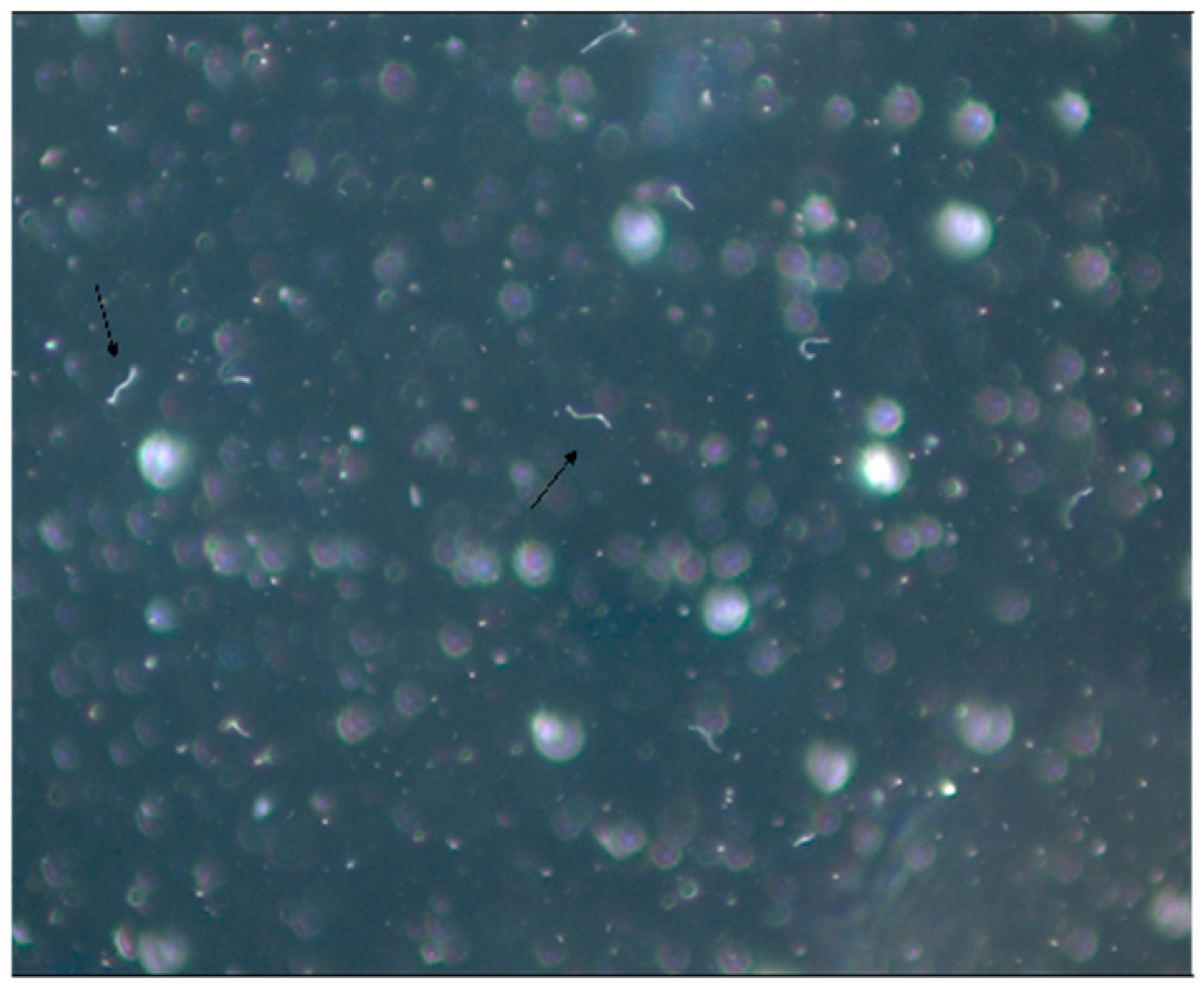
This second fieldwork conducted in Campo Grande County, state of Mato Grosso do Sul, confirmed the absence of ticks of the Ixodes ricinus complex, which are ticks usually involved as vectors in the transmission of LD in the Northern Hemisphere. It is important to note that vectors of complex I.ricinus are I. pacificus found in the USA, I. scapularis also observed in the USA, I. ricinus present in Europe and I. persulcatus existing in Asia. This research carried out in Campo Grande confirmed the absence of these ticks of genus Ixodes, transmitters of LD in Northern Hemisphere at-risk areas in Brazil. Surprisingly, for the first time, the fieldwork implicated the possible role of ticks of the genera Amblyomma and Rhipicephalus as vectors of Brazilian borreliosis. Isolation and culture of conventional spiral and mobile Borrelia sp were not possible, in spite of the presence of spirochete-like structures in cultures derived from animal blood, organs and ticks. It should be specified that such few mobile bacteria-like structures were also observed in human blood cultures, especially those suspected of having Brazilian LD (Figure 1).
Figure 1. Patient’s blood culture in SP4* medium viewed on dark field microscopy (1000×). Mantovani et al. (2007). * SP-4 medium is used for Spiroplasma culture.
1.7. Brazilian Lyme Disease-like Patients Display Low Immune Response to B. burgdorferi
Analysis of the first Brazilian LD-like cases showed a low humoral and cellular immune response to
B. burgdorferi when compared with North American patients at active disease stage
[14][15][16][17][18][19]. Brazilian patients always exhibit a low immune response to
B. burgdorferi at all disease stages, suggesting the presence of less immunogenic spirochete in the country.
Enzyme-linked immunosorbent assays (ELISA) performed with
B. burgdorferi G 39/40 exhibited low serological immune response in about 35% of Brazilian LD patients in the second stage of the disease. Additionally, Western blotting assay (WB) did not fulfill the diagnostic criteria suggested by Dresslet et al. (1993)
[20]. According to the authors, positive IgM and IgG immunoblotting must show two of the following bands: 18, 21, 28, 37, 41, 45, 58, 93 kDa, or five of the following molecular weight bands: 18, 21,28,30,39, 41, 45, 58, 66, 93 kDa, respectively. In contrast to these data, Brazilian patients, in general, express fewer bands and usually do not fulfil WB diagnostic criteria for LD
[21].
For lack of other options, a positive WB that showed the presence of two IgM or four IgG bands was considered , regardless of the molecular weight. It is important to note that this procedure matches the initial WB interpretation provided by the Tufts School of Medicine, Boston, in 1989
[22].
Despite these difficulties, when ELISA tests (IgG plus IgM) were added to the WB results, employing the HCFMUSP methodologies, positivity in 65% of Brazilian cases of LD-like illness could be observed . This low rate is still statistically significant when compared with the control group consisting of healthy individuals. Other matters must be taken into account regarding the interpretation of the ELISA test adopted in Brazil. In addition to its low sensitivity, it also shows low specificity since false positive reactions were reported in patients with other infectious and rheumatic diseases such as syphilis (IgM and IgG) and visceral leishmaniosis (IgG) and diffuse connective tissue diseases such as scleroderma (IgG), rheumatoid arthritis (IgM) and neurological autoimmune diseases
[2][15][18].
At the time, it was aksed that if the multiple laboratorial passages of the B. burgdorferi strain G39/40 in BSK II medium caused the loss of bacterial plasmids and antigens, causing minor ELISA and WB tests sensibility to identify Brazilian LD-like patients.
In this respect, the ELISA and WB tests using the European strains of
B. burgdorferi sensu lato were then performed , kindly provided by Dr Arno Artur Gustav Schönberg from the Federal Institute for Risk Assessment in Germany. However, it was noticed that ELISA and WB tests were conducted using antigens of the
B.garini strain 1B29,
B.afzelii strain 61BV1 and
B.burgdorferi sensu strictu strain 61BV3, which provided similar results when compared with North American
B. burgdorferi sensu stricto G39/40 strain
[23]. These results confirmed the low immune response of Brazilian LD-like patients to Borrelia antigens of North American or European origins.
2. Reasons Why the Brazilian Lyme Disease-like Zoonosis Was Named Baggio–Yoshinari Syndrome
In 1989, the HCFMUSP laboratory proposed to identify LD cases in Brazil. Over the years, typical cases of Borreliosis with erythema migrans (EM) and multisystem clinical involvement with arthritis, carditis, neurological and ocular symptoms have been detected in the country.
However, as the research progressed in Brazil, unexpected results were started to be observed. The main differences noticed between the Borreliosis found in Brazil and that of the Northern Hemisphere included the absence of the
Ixodes ricinus complex ticks in risk areas; failure to isolate and culture the etiological agent
B. burgdorferi in BSK II medium; low sensitivity and specificity of serological diagnostic tests (ELISA and WB) to identify suspected cases in Brazil; failure to identify flagellin and OspB genes by PCR
[24].
These divergences caused great confusion and suspicion around the research, and consequently, the existence of LD in Brazil was questioned. Generally, physicians and students are accustomed to studying a given scientific matter through consultation with books and scientific papers published in Medical Journals. However, as the knowledge evolved and studies were being published in the country, many serious disagreements emerged as the results gathered in Brazil were completely different from those accepted by scientists outside this country. Were it not for the respectability of the FMUSP as an institution and its researchers, the project of identifying Brazilian LD-like illness would have ceased prematurely.
Due to the findings that followed these contradictory results, which conflicted with the dogmas embraced by outside researchers on Lyme disease, the research data were never accepted for publication by International Journals. Reviewers ridiculed the possibility that Brazilian Borreliosis could be caused by ticks not belonging to the
Ixodes ricinus complex, did not acknowledge patients who did not fulfill the serological diagnostic criteria adopted by the Centers for Disease Control and Prevention (CDC) as well as the description of spirochete-like microorganisms; the absence of molecular biology positive cases, even after performing PCR tests with primers to identify the
Borrelia genus; reports of relapsing cases, even after antibiotic use as recommended by the CDC criteria. Therefore, at the time, it was asked what kind of disease was occurring in Brazil and how to confront it
[25][26][27].
As a consequence of these issues over time, this unusual tick-borne disease was named in many different ways, such as Brazilian Lyme disease, Brazilian Lyme disease-like illness, Lyme disease-simile illness, Infectious-reactive Lyme disease-like syndrome; and finally, Baggio–Yoshinari Syndrome (BYS)
[28].
The term Baggio–Yoshinari Syndrome (BYS), adopted in 2005, expresses a tribute to Prof Domingos Baggio, who died a few years after the beginning of the project, and also implies that a new Brazilian tick-borne disease, completely different from LD, despite the many clinical similarities with Lyme Borreliosis. In this regard, it deserves the attention of specific research to understand its etiological agent, its epidemiological transmission cycle, laboratory diagnosis, clinical manifestations and treatment. In fact, what was wished was to be stated that it was that Lyme disease does not exist in Brazil, except for imported cases, and to stop making comparisons with the disease we were used to seeing in the Northern Hemisphere. The choice of adopting the eponym Baggio–Yoshinari was not an act of self-referential. What it means is that all criticism and scientific mistakes must be attributed to both researchers.
At the beginning of the studies, it was thought that Brazilian Borreliosis could be similar to a disease known as STARI (Southern Tick Associated Rash Illness)
[29], identified in the south of the USA, characterized by the presence of a rash similar to the EM, without the appearance of systemic symptoms. It is caused by the spirochete
B. lonestari, isolated and cultured only in tick cells
[30]. BYS differs from STARI as Brazilian borreliosis causes systemic manifestations, and its etiological agent has remained uncultivable until the present day.
Currently, Brazilian physicians accept that BYS is an original clinical and laboratorial expression of B. burgdorferi infection, very distinct from that exhibited by Lyme disease. This important scientific knowledge is possibly related to the passage of spirochete through different ticks present in Northern Hemisphere and in Brazil, reflecting the influence of ecology and tick biodiversity on disease expression. Due to unknown reasons, possibly, B. burgdorferi infecting Brazilian vectors and reservoirs, present genotypic changes (mutations?), originating morphologically and antigenically modified spirochetes, adapted to survive in the geographical and biodiversity conditions.
According to the hypothesis, studies on BYS could help to explain why LD is not clearly reported in other regions of the Southern Hemisphere (Australia, Africa, New Zealand and South America), geographical areas with important biodiversity on ticks and reservoir distribution. A preliminary literature review indicated the absence of ticks from the complex Ixodes ricinus in these regions, suggesting that different ticks which do not belong to the genus Ixodes, similarly to what occurs in Brazil, could originate from exotic Borreliosis, different from typical LD found in USA, Europe and Asia. In this respect, research on Brazilian borreliosis could help to understand the many laboratorial and clinical expressions of spirochetosis caused by B. burgdorferi, according to tick and reservoir biodiversity found around the world, mainly in the Southern Hemisphere.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11080889