The significance of non-blood feeding cyclorrhaphan flies with some synanthropic, dipteran families (i.e., Calliphoridae, Sarcophagidae, and Muscidae) in transmitting pathogens to humans and their food sources needs a lot of focused research. People often overlook the small things in life. However, they can have a major impact on things that they have contacted. Synanthropic flies have been ignored in most epidemiological studies and models. An attempt has been made in this research to convince you that synanthropic flies can play and do play a major role in transmitting numerous pathogens to humans, domestic animals, and wildlife. The majority of zoonoses have been reported to take place between wildlife and various synanthropic flies. Prior to the origin of humans, insects evolved around 400 million years ago. They certainly had contact with the feces and mouth secretions of other animals. What took place people may never know but for certain, these flies were involved in the uptake and transmission of the numerous pathogens they acquired with their meal.
1. Introduction
As an anthropocentric culture, people tend to always look to the future, forgetting the past. Yet, early modern plague writers noted that one of the main organisms associated with impending plagues was flies
[1]. Recently, Balaraman et al.
[2] showed that SARS-CoV-2 could be mechanically transmitted by adult house flies, and this suggests that house flies might be more important in pandemics than previously thought. At the same time, current research efforts to identify causes or factors involved in predicting various emerging infectious diseases generally consider insect vectors as important and, presumably, they are referring to blood-sucking flies
[3][4], but they usually ignored transmission by synanthropic flies. How have synanthropic flies been ignored when recent studies (e.g., fly involvement in anthrax transmission) show they are extremely important as transmitters of pathogens? The famous surrealist painter, Salvador Dali, must have encountered synanthropic flies and even considered them important because he did some wonderful paintings of calliphorid adults
[5].
This research will attempt to influence your thinking about synanthropic flies in the lives of non-humans, as well as flies in early primate species. “It is likely that during centuries and until recently, the main route of simian pathogen transmission to humans was NHP (sic, non-human primates) hunting and wild meat consumption,”
[6]. In his popular book (i.e., Spillover), Quammen
[7] states “It (sic, a pathogen) can travel from one farm to another on a humid breeze”. More likely, the pathogen he is talking about can go from farm to farm via flying synanthropic flies. The researchers cannot fail to recognize the importance and the involvement of arthropods, especially the 3 cyclorrhaphan families discussed here, as potential transmitters of pathogens of non-human primates (NHP), humans, domestic animals and wildlife. Furthermore, these flies can serve as “fly driven contamination” sources or what Hugh-Jones and de Vos
[8] called a “multiplier hypothesis” whereby the flies can rapidly expand the size of the epizootic. In their review of the links and interfaces amongst wildlife, livestock, and human disease control, the authors
[9] only mentioned mosquitoes and ticks, ignoring some significant non-bloodfeeding Diptera discussed here.
Members of some countries often fail to realize that, in many countries, food is scarce, especially at certain times of the year. It is this scarcity of food or lack of food security that leads people to seek wild meat, whether it is hunted, found dead, or obtained somewhere in the world in a wet market. The risk of getting a pathogen is often not even considered. Finding something to eat is more important. This is what happened in 2011 in Chama, Zambia, when there were 511 human cases of anthrax (
Bacillus anthracis) infections resulting in 5 deaths because individuals ate meat from a dead hippopotamus
[10]. In addition to chronic food security issues, cultural habits often come into play. This happened in the spillover from the chimpanzee SIV to human HIV
[11] and is believed to have involved the cultural habit of eating bushmeat
[12][13][14]. In some countries, however, the researchers should not dismiss the possibility of fly-borne pathogens being transported to the foods eaten
[15]. More will be said about this later.
What Devaux et al.
[6] said in his above statement still leaves unanswered the question ‘Where do NHPs or other non-human animals, such as bats and birds, that are often eaten because of hunger or cultural/religious reasons obtain their microbes/parasites. Eating contaminated foods could lead to subsequent human infections, epidemics and even pandemics?’ In an interesting paper, Bennett et al.
[16], studying fruit bats, made the statement that there was a strong relationship between bats and arthropods. Important between this association is that bats are reported to house more zoonotic pathogens than all known species of mammals
[17]. This association with arthropods was also emphasized in the paper by Gessain
[18], who stated “Most of the persons included in this research were hunters of such NHPs, thus at high risk of contact with infected body fluids (blood, saliva, etc.) during hunting activities.” It is these dead animals, or their feces, that non-blood sucking flies of the calliphorid, sarcophagid, and muscid families are attracted to for egg laying and feeding on the blood and other body fluids, which they immediately dispatch into their crop organ and eventually use them for their own reproductive and/or metabolic strategies
[19][20]. Once fed, and when flies find another food source, or place to lay eggs, the crop fluids are either externally removed by regurgitation or bubbling or sent internally to the midgut for digestion. If the midgut route is taken, the pathogens exit by defecation. Both processes (oral regurgitation and defecation) might contain a pathogen/parasite infectious source. This is probably the scenario of what happened in the study by Echeverria et al.
[21] where they showed that adult flies, including house flies, accounted for numerous enteric pathogens causing diarrhea in the village of Ban Pong, Thailand. Caution to readers when seeing the term excretion in the literature because some authors use this term to define any product that exits either the mouth or anus of a fly. In this paper, the term excretion is not used, but emphasis is placed on regurgitation from the mouth and defecation from the anus.
2. What Is a Synanthropic Fly?
These undomesticated flies live in close association with and, in some way benefit from the association with humans. The literature reveals that almost all studies on synanthropic flies focus on a survey of flies associated with humans, the pathogens they carry, types of diseases associated with them, and the type of traps used to collect them. No paper was found on behavioral or physiological mechanisms that might connect these flies with humans or their domesticated animals.
The one physiological/behavior mechanism that might connect a fly species with humans is the ability of house flies to detect lactose. It is suggested that the common house fly,
M. domestica, evolved the ability to taste and respond to lactose while other flies do not have this ability, thus the house fly’s association with lactating animals. Instead of the smaller house fly, it is the larger calliphorids or sarcophagids
[22] that make better transfer agents. In the paper by Maldonado and Centeno
[23], the authors modified Mihályi’s danger index
[24] to include synanthropic information permitting them to discriminate between flies that were asynanthropic, hemisynanthropic, and eusynanthropic (
Figure 1). In that same paper, the authors state, “The idea of a direct relationship between body size and the capability of contamination and transmission of pathogens seems to be supported by several researchers around the world (Mariluis et al., 1989, Brown 1997, Graczyk et al., 1999, 2000, Fischer et al., 2001)”. If size is important, then for sure, it involves the crop organ. The bigger the fly, the more the crop can hold, and it holds more than the midgut. Mihályi
[24] did not consider the synanthropic index (SI) sufficiently informative, in a sanitary sense, and for this reason he made up a danger index keeping in mind 3 aspects of the behavioral and morphological characteristics of the insects: the undergoing infection, the passing infection and body size of the fly transmitter. Another important paper is that of Blacio et al.
[25], where they collected 2925 specimens of Calyptratae, but surprisingly, they did not collect any house flies. Nuroteva
[22] is an important paper to read where the author determines a synanthropic index for flies in Finland and provides a broad analysis of papers prior to his publication as to how different regions show different SI for the same species. Regardless of which synanthropic index is used to calculate which flies or animals are found in association with humans, more studies need to be done in areas where flies have proven to be involved in transmitting human or animal pathogens, such as anthrax or food-borne pathogens.
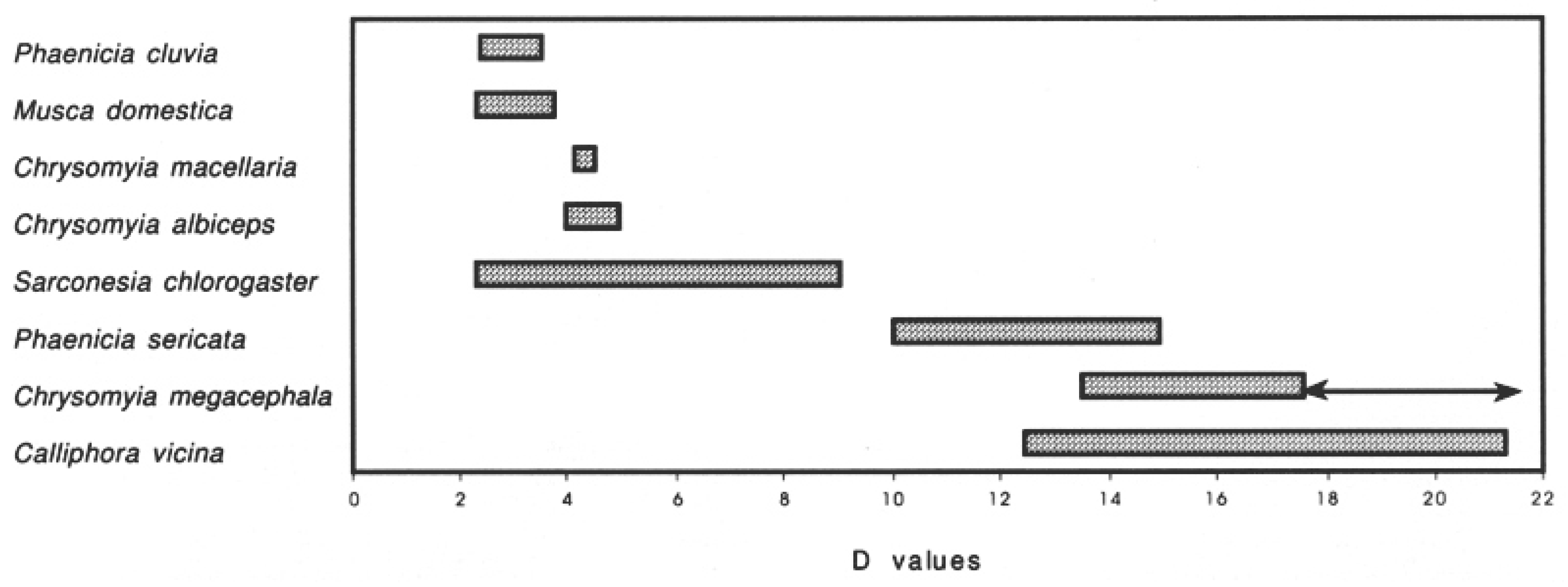
Figure 1. Taken from Maldonado and Centeno
[23] showing the danger index (D) for flies collected and the arrow line showing the changes for
Chrysomyia megacephala as considered now as hemisynanthropic (D = 19.43).
3. Were Synanthropic Flies Originally Associated with NHP and Other Animals?
The researcher searched for a term similar to synanthropic but refers to the fly association with NHP and early hominid species with little success. Based on the analysis of projected origin dates (house flies, 86–48 MYA; NHP, 85–55 MYA; hominids, 14 MYA), it is obvious that the three fly families (i.e., Calliphoridae, Sarcophagidae, and Muscidae) were present prior to the evolution of both hominids and humans, as the researchers know them. However, based on when they are believed to have evolved, it is also obvious that house flies and NHP occurred at about the same time. Spillar
[26] examined the diets of some muscoid flies and noted that for some larvae, the importance of lactose had not been examined, yet is essential to lactating animals. The importance of lactose for the now common, house fly,
M. domestica, however, would change possibly making it a very synanthropic species. Schnuch and Seebauer
[27], based on electrophysiological experiments of taste chemoreceptors, showed that adult house flies can taste lactose, while experiments with other flies failed to show a tarsal or labellar chemoreceptive response to lactose. The ability to taste lactose might have been a key factor in the co-evolution between the house fly and humans in the highly domesticated situation of humans using lactating animals for milk. However, house flies originally had close contact with groups of lactating animals and, only later after a gradual transition, did they maintain a close relationship with humans. Is there any reported evidence of adult house flies feeding on the lactose-based secretions of NHPs? Wiesmann
[28] examined the crops of field-caught house flies in stables and farmhouses and showed that the crops all contained lactose. Thus, a new food source for adult house flies. According to Wiesmann
[28],
Musca domestica ignores the nectar of flowers as an energy source, but searches for its food in human habitations and in stables of domestic animals
[28]. The literature is scarce as to house flies feeding on nectar or honeydew. What does house fly feed on in areas lacking or deficient in domesticated animals and, are the chemoreceptors of adult house flies in those areas still able to detect lactose? It is suggested that lactation may have originated from pre-mammal ancestors over 150 MYA
[29]. It is proposed that, in adaptation to the availability of milk in its environment,
Musca domestica acquired the ability to perceive, as well as to metabolize, lactose. More research needs to focus on the behavioral and physiological mechanisms of synanthropic flies.
One could ask if non-bloodsucking flies ever show a close association with groups of NHP and, would this put them in a position to pick up and transfer pathogens they house? The answer is yes, but a lot more research needs to be done. “Primate-associated flies were observed to move between groups of different species, suggesting that they could be involved in transmitting the yaws pathogen between species, even when NHPs are not found in mixed-species associations”
[30]. A recent report by Gogarten
[31] notes that by studying the fly and monkey associations that larger monkey groups harbored higher fly densities.
Figure 2. shows the close association between flies and a sleeping chimpanzee. Gogarten’s paper
[31] is a must-read if one wants to know more about whether NHPs have developed defensive behaviors against nuisance flies and what the researchers should call this close association with NHP.
Figure 2. Flies attracted to a sleeping chimpanzee. Photo courtesy of Fabian Leendeertz and the Tai Chimpanzee Project.
The researchers can also imagine that many of the current and known pathogens are being moved around amongst NHPs and other animals, including bats (50–60 MYA) and birds (modern birds, 150 MYA;
[32]). Did house flies co-evolve with humans, as the researchers know them, or was it present for interaction with NHPs? As previously stated, the house fly is reported to have evolved 48–86 MYA while
Homo sapiens, as the researchers know, entered the picture only 0.2 MYA. An important question is, what did
M. domestica feed on as adults prior to the presence of
Homo sapiens and, the use of milk from domestic animals? Free lactose is only present in lactating mammals and lactating animals had their origins in a pre-mammalian ancestor over 150 MYA
[29], which is a much longer period before the house fly is reported to evolve.
In an important study, Gogarten et al.
[30] examined, marked, and recaptured flies that remained with the monkey troops for 12 days and for up to 1.3 km of their movement. Of these flies, 45.8% were shown to be Muscidae, 35.4% Calliphoridae, and 8.3% Sarcophagidae. Sixty percent of the flies captured in feces were Muscidae. Can this behavior of flies following monkey troops be considered a forerunner of synanthropic behavior? It is well established that synanthropic flies are those flies that are closely associated with humans, as people know them. What do people know about the flies associated with other hominids, such as Neanderthal, Denisovans, and more recently the ghost hominid? Why did
M. domestica become the only truly synanthropic, non-blood feeding fly remains an academic issue but, which probably could be solved by doing gene mining and asking when the lactose receptor evolved in house flies?
Another question that needs to be answered is, which fly species contribute most to transmitting various pathogens? If you ask someone interested in food safety or domestic animal health, the answer would universally be the common house fly
[33]. Historically, and even to date, more research has been devoted to this fly because of its close association with humans, humans' domestic animals, and its feeding on the numerous types of filth associated with humans and domestic animals. An interesting and a must-read paper is by Maldonado and Centeno
[23] who attempted to answer the above question. They analyzed data for seven Calliphorid blowflies and the adult house fly to produce a synanthropic or danger index. They mention that previous workers proposed there was “… a direct relationship between body size and the capability of contamination and transmission of pathogens seem to be supported by several researchers around the world.” Their results did show that size was significantly important in having a high danger index. This might make sense if one considers the size of the crop of larger species and the number of infectious agents it could store. In addition to crop size, however, the rate of regurgitation should also be considered. Maldonado and Centeno
[23] proceeded, and based on their analysis, they concluded that 5 of the calliphorid blowflies were better suited as pathogen transfer agents over the house fly and, only one blowfly was lesser of a problem. This may seem like a surprising result but, is the house fly a major disseminator of pathogens in tropical rain forests? House fly is rarely reported in tropical rainforest settings, especially with NHP.
This entry is adapted from the peer-reviewed paper 10.3390/insects13090776