Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Straight-chain aliphatic aldehydes were repeatedly detected in the breath of patients suffering from lung diseases using a variety of methods, such as mass spectrometry, ion mobility spectrometry, or electro-chemical sensors.
- aldehydes
- lipid peroxidation
- oxidative stress
- breath analysis
- volatile organic compounds
- lung diseases
1. Introduction
More than seven million patients die each year from lung diseases, putting enormous socioeconomical burdens on society and health care systems [1]. An early diagnosis may help to initiate treatment at early disease stages to improve treatment outcomes. Identification of biomarkers enabling early diagnosis and treatment is therefore of considerable interest.
Breath analysis could provide rapid, repeatable, and non-invasive diagnosis of numerous diseases by the detection of disease-specific alterations of exhaled volatile organic compounds (VOCs). Although usually patterns of changes in the composition of exhaled air (also referred to as the “exhalome”) help to identify diseased patients, some specific compounds were repeatedly found as potential markers of damage.
A good example is the increased exhalation of straight-chain aliphatic aldehydes in patients suffering from lung diseases. The well-known generation process by lipid peroxidation [2][3][4], high volatility, and good detectability make them interesting candidates as biomarkers to diagnose and monitor progress of lung diseases; especially, since exhaled aldehydes can be measured at the point of care by detection methods such as ion mobility spectrometry and electrochemical sensors [4][5].
2. Origin of Straight-Chain Aliphatic Aldehydes
Aldehydes are ubiquitous compounds found in nature and are part of our daily life. They are highly reactive and consist of a carbonyl group attached to at least one hydrogen atom. They are represented as R-CHO, where R is an attached group, either aromatic or aliphatic. Researchers focused on C2 to C10 straight-chain aliphatic aldehydes, as listed in Table 1.
Table 1. Overview of straight-chain aliphatic aldehydes.
| Aldehyde | Chain Length | Structural Formula |
|---|---|---|
| Ethanal | C2 | 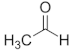 |
| Propanal | C3 | 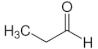 |
| Butanal | C4 | 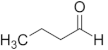 |
| Pentanal | C5 | 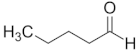 |
| Hexanal | C6 | 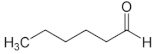 |
| Heptanal | C7 | 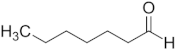 |
| Octanal | C8 | 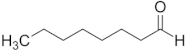 |
| Nonanal | C9 | 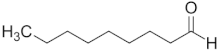 |
| Decanal | C10 |  |
Major sources for exogenous exposure to aldehydes are biomass and fossil fuel combustion, vehicle exhaust, power plants and wood burning fumes. For example, acetaldehyde and formaldehyde can be frequently detected in the surrounding air. However, smoking and the intake of alcohol are also major causes for exposure to aldehydes [6].
A major endogenous source for aldehyde generation is lipid peroxidation which is triggered by oxidative stress [7]. In healthy individuals a balance between oxidative and antioxidative mechanisms exists. When this balance is disturbed by diseases or injuries, reactive oxygen species and free radicals cause damage. Radicals oxidize and degrade polyunsaturated fatty acids of lipid membranes—a process called lipid peroxidation (Figure 1) [7][8].
Straight-chain aliphatic aldehydes are some of the most abundant products of lipid peroxidation [2][3][4][9] and are exhaled in the lower parts-per-billion (ppb) concentration range [10][11]. Exhaled aldehydes were thus repeatedly investigated as volatile biomarkers of lipid peroxidation-inducing diseases.
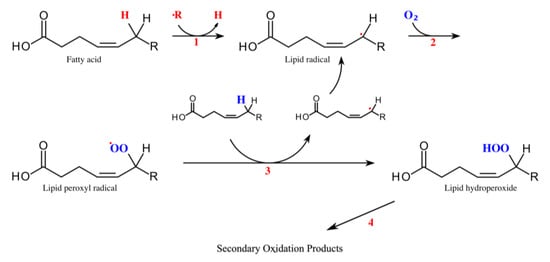
Figure 1. Lipid peroxidation. Modified from [12]. Two steps are essential for lipid peroxidation—initiation (1) and propagation (2). During initiation, a hydrogen atom is removed from the fatty acid forming a lipid radical (1). This can happen through enzymatic reactions from lipoxygenases, hydroperoxide-lyases and peroxygenases or by non-enzymatic processes. During propagation, the lipid radical reacts with oxygen which produces a peroxyl radical (2). In the next step the peroxyl radical reacts with another unsaturated lipid. It abstracts a hydrogen atom to form a hydroperoxide radical and a new lipid radical (3). Hydroperoxide radicals are unstable and quickly react to form other radicals and secondary products. In further cyclization reactions and cleavages, different compounds are produced including straight-chain aliphatic aldehydes (4) [8].
3. Detection Methods for Exhaled Aldehydes
Several methods were used to detect straight-chain aliphatic aldehydes in breath. Researchers will present a comprehensible overview on previously used methods in the following. Readers that are interested in an in-depth review on detection methods for volatile organic compounds are referred to the excellent methodological review by Buszewski et al. [13].
In general, gas chromatography–mass spectrometry (GC-MS) is considered the gold standard for the measurement of volatile organic compounds in breath. Large GC-MS databases enable the exact identification of an analyte according to retention time and molecular mass [13][14]. Given the universal applicability, several studies used GC-MS to detect aldehydes in breath. Further mass spectrometry methods, such as selected ion flow tube–mass spectrometry (SIFT-MS) or time of flight–mass spectrometry (TOF-MS) allow rapid real time measurements of exhaled aldehydes [13][15][16].
A downside of mass spectrometry systems is the bulky and expensive setup making point-of-care applications infeasible. Therefore, more portable systems were previously used for aldehyde detection in breath. For example, multi-capillary coupled–ion mobility spectrometry (MCC-IMS) can be applied at point-of-care [13][17], and has been used to detect aldehydes in exhaled breath in experimental and clinical settings [4][18][19].
Due to recent findings identifying aldehydes as potential breath biomarkers, electrochemical sensors were developed to further simplify point-of-care application. Obermeier et al. developed a combined sensor for aldehydes, carbon monoxide and nitric oxide and showed feasibility of continuous aldehyde monitoring in pigs [5]. In addition, the sensor enabled the identification of patients suffering from diabetes or lung cancer in a first pilot study [5]. Most recently, a zinc oxide nanowire sensor was developed for the detection of aldehydes down to 0.6 ppm which still needs optimization [20], as exhaled aldehydes are usually exhaled in the lower ppb concentration range [11]. Apart from good applicability for point-of-care analysis, specificity of these sensors for aldehydes remains unclear. As breath contains about 1500 volatile organic compounds, cross reactions of electrochemical sensors are likely [21]. There is thus a further need to develop and optimize point-of-care methods for aldehyde sensing.
4. Aldehyde Exhalation and Lung Cancer
Cancer is a leading cause of death with lung, breast and colorectal cancer contributing the most [22]. Early detection is essential to improve survival by initiation of treatments at early stages.
Screening methods for lung cancer have been debated for many years. Several biomarkers in blood and sputum have been investigated including DNA, RNA, circulating tumor cells, proteins and autoantibodies [23]. Currently, there is no established molecular biomarker used in clinical practice for early detection of lung cancer. Known markers, such as NSE, CEA and CA125 have poor sensitivity and specificity [24]. Combinations of biomarkers might improve the diagnostic value but larger multi-centric validation studies are pending [25][26].
Chest x-ray as a screening method showed no reduction in lung cancer deaths [27][28]. In addition, sensitivity of about 80% was reported in a recent review with almost 20% of lung cancer patients not being detected by chest x-rays [29].
Crucial for today’s screening was a study conducted by the National Lung Screening Trial Research Team. 53,454 patients were enrolled in a large multi-center study comparing the diagnostic value of a conventional chest x-ray versus a low dose computed tomography (LDCT). LDCT resulted in a reduction of mortality of up to 20% in high-risk patients [30]. The sensitivity for detecting lung cancer by LDCT is greater than 80% for which reason LDCT is the primary screening method for lung cancer in various countries despite risks of radiation exposure and overdiagnosis [31].
In contrast to the above-mentioned diagnostic methods, an optimal screening tool should be fast, cost-effective, and preferably non-invasive. All these requirements are provided by breath analysis.
“Cancer is a large group of diseases that can start in almost any organ or tissue of the body when abnormal cells grow uncontrollably, go beyond their usual boundaries to invade adjoining parts of the body and/or spread to other organs“ is the definition for cancer by the World Health Organization [32]. The “uncontrollably” “invasive” growth is accompanied by high metabolic activity. The increased metabolic activity resulting from the uncontrolled and fast growth leads to an increased production of reactive oxygen species, for instance, during oxidative phosphorylation in mitochondria, increased enzymatic activity and modified metabolism in cancer cells [33][34]. Consistently, increased concentrations of aldehydes as products of oxidative stress were detected in the headspace of cultured lung cancer cells [3], and increased aldehyde exhalation was repeatedly reported in lung cancer patients.
As one of the first in 2010, Fuchs et al. measured the concentrations of aldehydes in 12 lung cancer patients, 12 healthy smokers and 12 non-smoking healthy subjects. Exhaled concentrations of hexanal, pentanal, octanal and nonanal were significantly higher in lung cancer patients than in smoking or non-smoking healthy individuals. Propanal, butanal, heptanal and decanal concentrations did not differ between the groups [35].
In the same year, Poli et al. published results from measurements of aldehydes in exhaled breath of 40 lung cancer patients compared to 38 healthy individuals. They measured the exhaled concentrations of C3-C9 aldehydes. All measured aldehydes were significantly higher in the ex- and non-smoking lung cancer patients compared to controls, except for propanal, which was characteristic for smoking lung cancer patients. For example, the median exhaled concentration of butanal in lung cancer patients was more than twice as high as in controls (10.8 pM vs. 26.2 pM) and hexanal concentration was three times greater in lung cancer patients compared to controls (10.3 pM vs. 38.1 pM). By using this set of aldehydes, 90% of lung cancer patients and 92% of controls were classified correctly [36].
Buszewski et al. measured exhaled concentrations of aldehydes in 44 healthy individuals and 29 lung cancer patients. Propanal was increased in lung cancer patients and again in smokers. Butanal was significantly increased in lung cancer patients compared to healthy individuals and smokers. Other aldehydes were not measured [37].
Handa et al. analyzed 115 different volatile organic compounds measured in 50 patients with lung cancer and 39 healthy controls. Ten peaks were significantly higher in lung cancer patients including hexanal, heptanal and nonanal. Nonanal additionally allowed the differentiation between squamous cell carcinoma and adenocarcinoma [38]. Consistently, Baumbach et al. found increased concentrations of nonanal obtained during bronchoscopy in lung cancer patients [39].
Corradi et al. and Ulanowska et al. conducted two of the largest studies that found increased aldehyde exhalation in 138 and 137 lung cancer patients, respectively. Corradi et al. measured increased concentrations of heptanal in lung cancer patients. Interestingly, exhaled concentrations of other aldehydes were not increased [40]. One reason for this might be the composition of the control group which included patients with lung diseases other than cancer. As outlined in the following chapters, inflammatory and infectious lung diseases influence aldehyde exhalation which might have diminished the difference of aldehyde exhalation in comparison to lung cancer patients. In contrast, Ulanowska et al. found increased exhaled concentrations of propanal in lung cancer patients compared to healthy individuals. Furthermore, pentanal and hexanal were only detectable in cancer patients but not in healthy individuals [41].
Finally, a recently published investigation included 157 lung cancer patients and 368 healthy individuals. Pentanal, hexanal, heptanal, octanal, nonanal and decanal exhalation was increased in lung cancer patients. Breath sampling was performed immediately before surgery to minimize impact of external factors such as environmental contaminations or prior food intake [42].
In contrast to the above presented findings, some studies could not show increased aldehyde exhalation in lung cancer patients. For example, Callol-Sanchez et al. screened 81 lung cancer patients and 83 healthy control patients explicitly for exhaled aldehydes but did not detect a difference [43].
Several reasons might explain the different findings throughout the literature. First is the subject-specific influence on aldehyde exhalation, as sex-related differences in propanal exhalation were reported [44]. Thus, results might be biased by unbalanced baseline characteristics of the assessed study populations. For example, most healthy subjects included in the study of Fuchs et al. were between 20 and 30 years old, whereas all lung cancer patients were older than 50 years [35]. Larger studies on exhaled aldehydes as biomarkers for lung cancer with more than 100 subjects, providing more balanced baseline characteristics, are still rare [40][41][42]. Future studies may thus focus on influences of baseline characteristics such as age, sex, or comorbidities on aldehyde exhalation.
Second, different sampling methods were used throughout the presented studies. Tedlar bags were mostly used but also Bio-Voc systems were used. Probands exhale into these systems and the breath sample is transferred to the respective analysis device. Although made from inert materials, it was shown that used Tedlar bags may release volatile organic compounds from previous usage which highlights the interactions between sampling material and analytes [45]. Furthermore, Tedlar bag samples usually contain mixed exhaled air as opposed to Bio-Voc samples which mostly contain alveolar air. Thus a previous study found 137 VOCs with Tedlar bags compared to only 47 VOCs with the Bio-Voc system [46].
Third, contaminations from the surrounding, diet and medication may considerably alter the results from breath analysis. For example, Kischkel et al. found significantly increased exhaled aldehyde concentrations in lung cancer patients, which after correction for inspired concentrations was no longer significant [44]. Furthermore, previous exposition to fumes or disinfectants present in the hospital environment alters the composition of breath aldehydes, as for example propanal is a typical ingredient of disinfectants [47][48]. To minimize potential influences from diet and environmental contamination, sampling in the perioperative setting could thus be favorable [42].
Finally, different statistical methods and algorithms might produce different statistical significances. For example, Poli et al. performed in addition to analysis of variance between groups a multivariate analysis using a structure matrix and cross-validation and implementing factor scores for establishing a predictive algorithm [36]. Ulanowska et al. used discriminant analysis and a CHAID tree to test for statistical significance [41], whereas Handa et al., for example, didn’t use predictive models at all [38].
In summary, several studies found increased aldehyde exhalation in lung cancer patients. For assessing the usefulness of aldehydes as biomarkers for lung cancer, future studies should have standardized breath sampling methods, use rather large study populations, and eliminate contaminants from the surroundings. Finally, it remains unclear whether breath analysis is useful to screen for early disease stages, as current studies only included patients with lung cancer already diagnosed with established diagnostics potentially missing early stages.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27165258
References
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021.
- Yoshino, K.; Sano, M.; Jujita, M.; Tomita, I. Production of aliphatic aldehydes on peroxidation of various types of lipids. Chem. Pharm. Bull. 1991, 39, 1788–1791.
- Shestivska, V.; Rutter, A.V.; Sulé-Suso, J.; Smith, D.; Španěl, P. Evaluation of peroxidative stress of cancer cells in vitro by real-time quantification of volatile aldehydes in culture headspace. Rapid Commun. Mass Spectrom. 2017, 31, 1344–1352.
- Müller-Wirtz, L.M.; Kiefer, D.; Ruffing, S.; Brausch, T.; Hüppe, T.; Sessler, D.I.; Volk, T.; Fink, T.; Kreuer, S.; Maurer, F. Quantification of Volatile Aldehydes Deriving from In Vitro Lipid Peroxidation in the Breath of Ventilated Patients. Molecules 2021, 26, 3089.
- Obermeier, J.; Trefz, P.; Wex, K.; Sabel, B.; Schubert, J.K.; Miekisch, W. Electrochemical sensor system for breath analysis of aldehydes, CO and NO. J. Breath Res. 2015, 9, 016008.
- Sinharoy, P.; Mcallister, S.L.; Vasu, M.; Gross, E.R. Environmental Aldehyde Sources and the Health Implications of Exposure. Aldehyde Dehydrog. 2019, 1193, 35–52.
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438.
- Clemente, S.M.; Martínez-Costa, O.H.; Monsalve, M.; Samhan-Arias, A.K. Targeting Lipid Peroxidation for Cancer Treatment. Molecules 2020, 25, 5144.
- Ratcliffe, N.; Wieczorek, T.; Drabińska, N.; Gould, O.; Osborne, A.; De Lacy Costello, B. A mechanistic study and review of volatile products from peroxidation of unsaturated fatty acids: An aid to understanding the origins of volatile organic compounds from the human body. J. Breath Res. 2020, 14, 034001.
- McCartney, M.M.; Thompson, C.J.; Klein, L.R.; Ngo, J.H.; Seibel, J.D.; Fabia, F.; Simms, L.A.; Borras, E.; Young, B.S.; Lara, J.; et al. Breath carbonyl levels in a human population of seven hundred participants. J. Breath Res. 2020, 14, 046005.
- Huang, J.; Kumar, S.; Hanna, G.B. Investigation of C3-C10 aldehydes in the exhaled breath of healthy subjects using selected ion flow tube-mass spectrometry (SIFT-MS). J. Breath Res. 2014, 8, 037104.
- WikiCommons Lipid Peroxidation. Available online: https://commons.wikimedia.org/wiki/File:Mechanismus_der_Lipidperoxidation.svg (accessed on 11 April 2022).
- Buszewski, B.; Grzywinski, D.; Ligor, T.; Stacewicz, T.; Bielecki, Z.; Wojtas, J. Detection of volatile organic compounds as biomarkers in breath analysis by different analytical techniques. Bioanalysis 2013, 5, 2287–2306.
- Kim, K.H.; Jahan, S.A.; Kabir, E. A review of breath analysis for diagnosis of human health. Trends Anal. Chem. 2012, 33, 1–8.
- Sauer, S.; Kliem, M. Mass spectrometry tools for the classification and identification of bacteria. Nat. Rev. Microbiol. 2010, 8, 74–82.
- Singhal, N.; Kumar, M.; Kanaujia, P.K.; Virdi, J.S. MALDI-TOF mass spectrometry: An emerging technology for microbial identification and diagnosis. Front. Microbiol. 2015, 6, 791.
- Fink, T.; Baumbach, J.I.; Kreuer, S. Ion mobility spectrometry in breath research. J. Breath Res. 2014, 8, 027104.
- Müller-Wirtz, L.M.; Kiefer, D.; Maurer, F.; Floss, M.A.; Doneit, J.; Hüppe, T.; Shopova, T.; Wolf, B.; Sessler, D.I.; Volk, T.; et al. Volutrauma Increases Exhaled Pentanal in Rats: A Potential Breath Biomarker for Ventilator-Induced Lung Injury. Anesth. Analg. 2021, 133, 263–273.
- Ruzsanyi, V.; Baumbach, J.I.; Sielemann, S.; Litterst, P.; Westhoff, M.; Freitag, L. Detection of human metabolites using multi-capillary columns coupled to ion mobility spectrometers. J. Chromatogr. A 2005, 1084, 145–151.
- Olarve, R.S.; Dela Torre, H.M.; Foronda, J.R.; Santos, M.G.; Sajor, N.J.; Lopez, T.B.; Haygood, K.J.; Santos, G.N. Aldehyde Gas Detection using Nanostructured Zno-based Gas Sensor fabricated via Horizontal Vapor Phase Growth Technique. J. Phys. Conf. Ser. 2021, 2015, 012102.
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.D.L. A literature survey of all volatiles from healthy human breath and bodily fluids: The human volatilome. J. Breath Res. 2021, 15, 034001.
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33.
- Dama, E.; Colangelo, T.; Fina, E.; Cremonesi, M.; Kallikourdis, M.; Veronesi, G.; Bianchi, F. Biomarkers and Lung Cancer Early Detection: State of the Art. Cancers 2021, 13, 3919.
- Sun, J.; Chen, X.; Wang, Y. Comparison of the diagnostic value of CEA combined with OPN or DKK1 in non-small cell lung cancer. Oncol. Lett. 2020, 20, 3046–3052.
- Yang, Q.; Zhang, P.; Wu, R.; Lu, K.; Zhou, H. Identifying the Best Marker Combination in CEA, CA125, CY211, NSE, and SCC for Lung Cancer Screening by Combining ROC Curve and Logistic Regression Analyses: Is It Feasible? Dis. Markers 2018, 2018, 2082840.
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in lung cancer screening: Achievements, promises and challenges. J. Thorac. Oncol. 2019, 14, 343–357.
- Kubik, A.; Parkin, D.M.; Khlat, M.; Erban, J.; Polak, J.; Adamec, M. Lack of benefit from semi-annual screening for cancer of the lung: Follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int. J. cancer 1990, 45, 26–33.
- Fontana, R.S.; Sanderson, D.R.; Woolner, L.B.; Taylor, W.F.; Miller, W.E.; Muhm, J.R. Lung cancer screening: The Mayo program. J. Occup. Med. 1986, 28, 746–750.
- Bradley, S.H.; Grice, A.; Neal, R.D.; Abraham, S.; Rodriguez Lopez, R.; Shinkins, B.; Callister, M.E.J.; Hamilton, W.T. Sensitivity of chest X-ray for detecting lung cancer in people presenting with symptoms: A systematic review. Br. J. Gen. Pract. 2019, 69, E827–E835.
- Team, T.N.L.S.T.R. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409.
- Jonas, D.E.; Reuland, D.S.; Reddy, S.M.; Nagle, M.; Clark, S.D.; Weber, R.P.; Enyioha, C.; Malo, T.L.; Brenner, A.T.; Armstrong, C.; et al. Screening for Lung Cancer with Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. J. Am. Med. Assoc. 2021, 325, 971–987.
- WHO. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 11 April 2022).
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642.
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496.
- Fuchs, P.; Loeseken, C.; Schubert, J.K.; Miekisch, W. Breath gas aldehydes as biomarkers of lung cancer. Int. J. Cancer 2010, 126, 2663–2670.
- Poli, D.; Goldoni, M.; Corradi, M.; Acampa, O.; Carbognani, P.; Internullo, E.; Casalini, A.; Mutti, A. Determination of aldehydes in exhaled breath of patients with lung cancer by means of on-fiber-derivatisation SPME-GC/MS. J. Chromatogr. B 2010, 878, 2643–2651.
- Buszewski, B.; Ligor, T.; Jezierski, T.; Wenda-Piesik, A.; Walczak, M.; Rudnicka, J. Identification of volatile lung cancer markers by gas chromatography-mass spectrometry: Comparison with discrimination by canines. Anal. Bioanal. Chem. 2012, 404, 141–146.
- Handa, H.; Usuba, A.; Maddula, S.; Rg, J.; Baumbach, I.; Mineshita, M.; Miyazawa, T. Exhaled Breath Analysis for Lung Cancer Detection Using Ion Mobility Spectrometry. PLoS ONE 2014, 9, e114555.
- Baumbach, J.I.; Maddula, S.; Sommerwerck, U.; Besa, V.; Kurth, I.; Bödeker, B.; Teschler, H.; Freitag, L.; Darwiche, K. Significant different volatile biomarker during bronchoscopic ion mobility spectrometry investigation of patients suffering lung carcinoma. Int. J. Ion. Mobil. Spectrom. 2011, 14, 159–166.
- Corradi, M.; Poli, D.; Banda, I.; Bonini, S.; Mozzoni, P.; Pinelli, S.; Alinovi, R.; Andreoli, R.; Ampollini, L.; Casalini, A.; et al. Exhaled breath analysis in suspected cases of non-small-cell lung cancer: A cross-sectional study. J. Breath Res. 2015, 9, 27101.
- Ulanowska, A.; Kowalkowski, T.; Trawińska, E.; Buszewski, B. The application of statistical methods using VOCs to identify patients with lung cancer. J. Breath Res. 2011, 5, 046008.
- Wang, P.; Huang, Q.; Meng, S.; Mu, T.; Liu, Z.; He, M.; Li, Q.; Zhao, S.; Wang, S.; Qiu, M. Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study. EClinicalMedicine 2022, 47, 101384.
- Callol-Sanchez, L.; Munoz-Lucas, M.A.; Gomez-Martin, O.; Maldonado-Sanz, J.A.; Civera-Tejuca, C.; Gutierrez-Ortega, C.; Rodriguez-Trigo, G.; Jareno-Esteban, J. Observation of nonanoic acid and aldehydes in exhaled breath of patients with lung cancer. J. Breath Res. 2017, 11, 26004.
- Kischkel, S.; Miekisch, W.; Sawacki, A.; Straker, E.M.; Trefz, P.; Amann, A.; Schubert, J.K. Breath biomarkers for lung cancer detection and assessment of smoking related effects—Confounding variables, influence of normalization and statistical algorithms. Clin. Chim. Acta 2010, 411, 1637–1644.
- Ghosh, S.; Kim, K.-H.; Sohn, J.R. Some insights into analytical bias involved in the application of grab sampling for volatile organic compounds: A case study against used Tedlar bags. Sci. World J. 2011, 11, 2160–2177.
- Berna, A.Z.; Schaber, C.L.; Bollinger, L.B.; Mwale, M.; Mlotha-Mitole, R.; Trehan, I.; Odom John, A.R. Comparison of breath sampling methods: A post hoc analysis from observational cohort studies. Analyst 2019, 144, 2026.
- Koureas, M.; Kirgou, P.; Amoutzias, G.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Target Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Discrimination from Other Pulmonary Diseases and Healthy Persons. Metabolites 2020, 10, 317.
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Clinical Medicine Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2021, 10, 32.
This entry is offline, you can click here to edit this entry!
