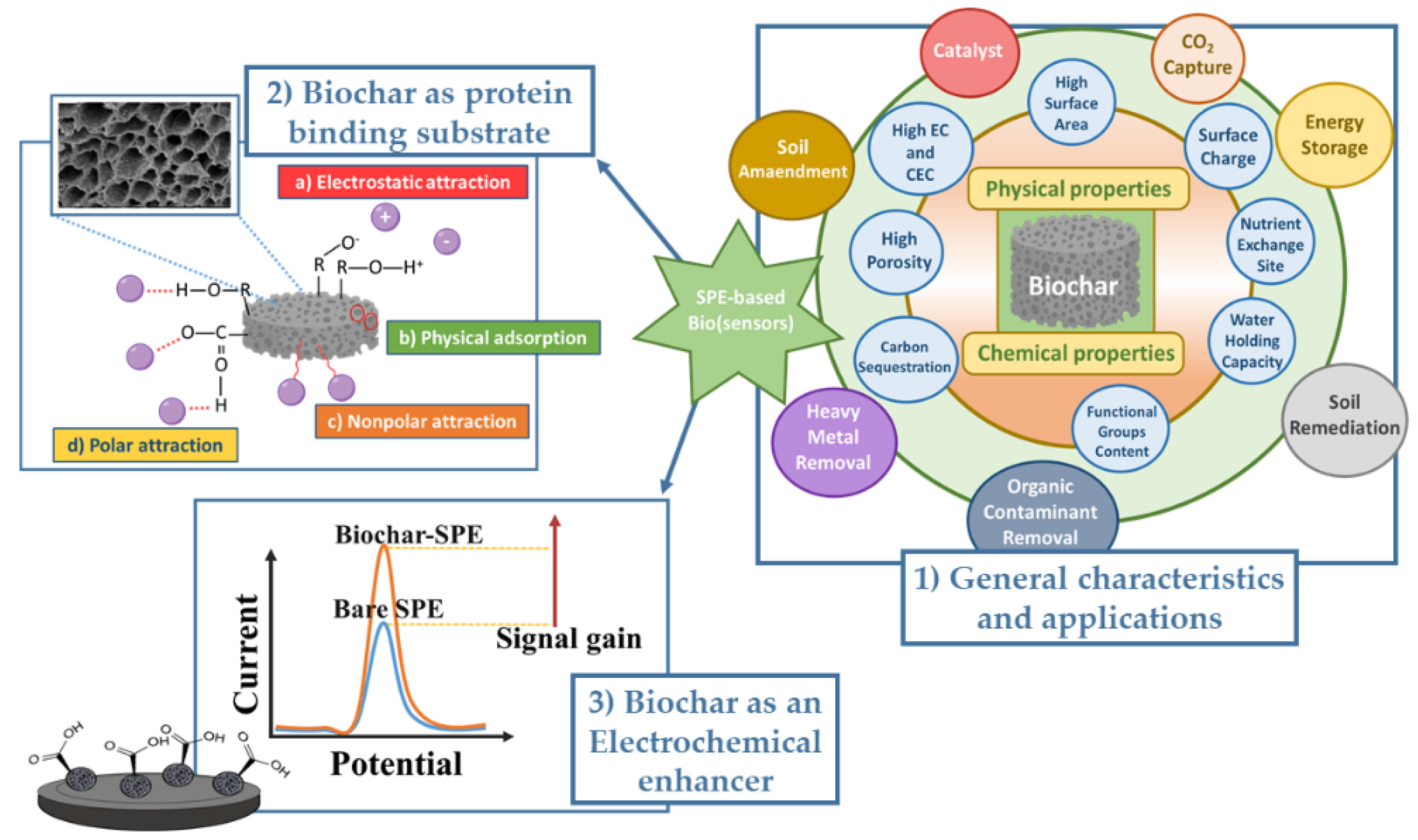
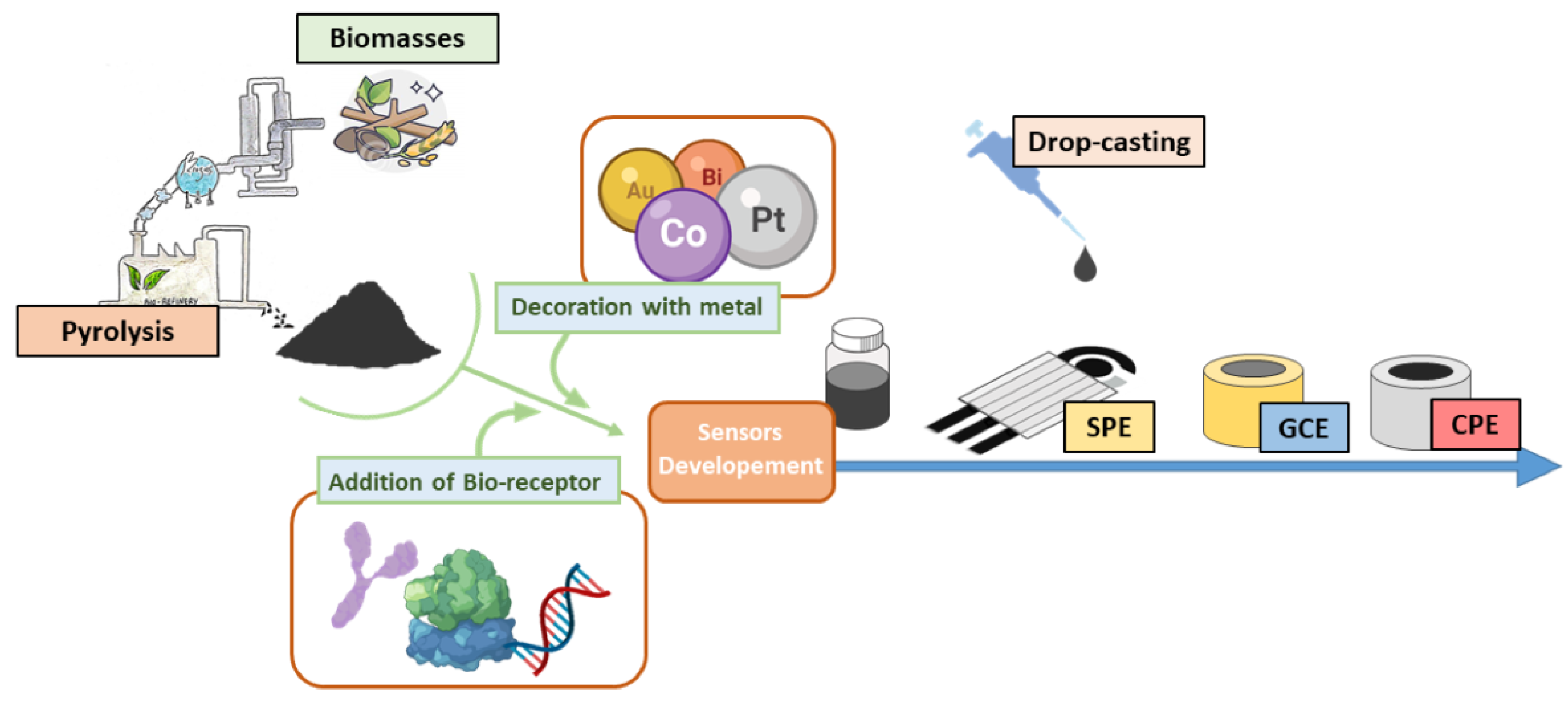
Biochar is a pyrolytic material with several environmental benefits such as reducing greenhouse gas emissions, sequestering atmospheric carbon and contrasting global warming. It has moved to the forefront for its conductivity and electron transfer properties, finding applications in the fabrication of electrochemical platforms. In this field, researchers have focused on low-cost biomass capable of replacing more popular and expensive carbonaceous nanomaterials (i.e., graphene, nanotubes and quantum dots) in the realization of sensitive cost-effectiveness and eco-friendly electrochemical tools.
- biochar-modified screen-printed devices
- sustainable sensors
- eco-friendly materials
1. Introduction
2. Application of Biochar in Electrochemical Platforms
2.1. Carbon-Paste and Glassy-Carbon Electrodes
| Feedstock | Electrode | Analyte | LOD (nM) |
Linear Range (M) |
Real Sample Matrix |
Reference |
|---|---|---|---|---|---|---|
| Castor oil cake |
Biochar-CPE | Cd Pb |
69 (Cd) 9.8 (Pb) |
(0.03–5.0) × 10−5 (0.001–5.0) × 10−5 |
wastewater | [39] |
| Castor oil cake |
HgND-Biochar-CPE | Zn | 170 | (0.07–3) × 10−5 | collyrium and ointment |
[40] |
| Castor oil cake |
Bi-nano-Biochar- CPE |
Pb | 1.41 | (0.005–1) × 10−3 | Overglaze decorated ceramic dishes |
[41] |
| Castor oil cake |
Biochar-CPE | Cu | 400 | (0.1–3.1) × 10−5 | Vodka, cachaça, gin and tequila |
[42] |
| Castor oil cake |
Sbmicro-Biochar- CPE |
Paraquat | 34 | (0.03–1.0) × 10−6 | natural water coconut water |
[43] |
| Castor oil cake |
Biochar-CPE | Isoniazid | 63 | (0.1–1.0) × 10−5 | Synthetic human urine |
[44] |
| Castor oil cake |
Biochar-CPE | Methyl Parathion |
39 | (0.01–170) × 10−6 | Drinking water and fruit juices |
[45] |
| Sugarcane bagasse |
BioNano-GCE | 17 β- estradiol |
11.3 | (0.05–20) × 10−6 | Groundwater | [46] |
| Kiwi skin | ZnCl2/Biochar GCE | AA Dopamine UA |
20 160 110 |
(0.05–200) × 10−6 (2–2000) × 10−6 (1–2500) × 10−6 |
Human urine | [47] |
| Dandelion pappus |
Npc/GCE | Try | 30 | (1–103) × 10−6 | Commercial amino acid injection and Fetal calf serum |
[48] |
| Bamboo | BioNC-GCE | Tmx | 220 | (0.5–35) × 10−7 | Spinach, Rice, Pear, Red soil, Tap water, Farmland water, River water, Lake water |
[49] |
| Water Hyacinth |
Biochar-rGO- CPE |
Cbz | 2.3 | (30–900) × 10−8 | Orange juice, Lettuce leaves, Drinking water, Wastewater |
[50] |
| Castor oil cake |
Ac-biochar-CPE | Caffeic acid | 30.9 | (1.0–3000) × 10−6 | White, Rose and Red wine |
[51] |
| Castor oil cake |
Ni/biochar-CPE | Glucose | 137 | (5–100) × 10−6 | Human saliva Blood serum |
[52] |
| Pumpkin stems |
Biochar-GCE | AA Dopamine UA |
2300 30 510 |
(30–95) × 10−6 (1–65) × 10−6 (2–230) × 10−6 |
SH and Human blood serum |
[53] |
| Bamboo fungus |
Biochar-GCE | Bis-A | 1068 | (0.02–10) × 10−6 | Groundwater | [54] |
| Kelp | Act-biochar-GCE | Acp | 4 | (2.5–2000) × 10−6 | Human serum And Medical tablet |
[55] |
| Feedstock | Electrode | Analyte | LOD (nM) |
Linear Range (M) |
Real Sample Matrix |
Reference |
|---|---|---|---|---|---|---|
| Castor oil cake |
Biochar-CPE | Cd Pb |
69 (Cd) 9.8 (Pb) |
(0.03–5.0) × 10−5 (0.001–5.0) × 10−5 |
wastewater | [39] |
| Castor oil cake |
HgND-Biochar-CPE | Zn | 170 | (0.07–3) × 10−5 | collyrium and ointment |
[40] |
| Castor oil cake |
Bi-nano-Biochar- CPE |
Pb | 1.41 | (0.005–1) × 10−3 | Overglaze decorated ceramic dishes |
[41] |
| Castor oil cake |
Biochar-CPE | Cu | 400 | (0.1–3.1) × 10−5 | Vodka, cachaça, gin and tequila |
[42] |
| Castor oil cake |
Sbmicro-Biochar- CPE |
Paraquat | 34 | (0.03–1.0) × 10−6 | natural water coconut water |
[43] |
| Castor oil cake |
Biochar-CPE | Isoniazid | 63 | (0.1–1.0) × 10−5 | Synthetic human urine |
[44] |
| Castor oil cake |
Biochar-CPE | Methyl Parathion |
39 | (0.01–170) × 10−6 | Drinking water and fruit juices |
[45] |
| Sugarcane bagasse |
BioNano-GCE | 17 β- estradiol |
11.3 | (0.05–20) × 10−6 | Groundwater | [46] |
| Kiwi skin | ZnCl2/Biochar GCE | AA Dopamine UA |
20 160 110 |
(0.05–200) × 10−6 (2–2000) × 10−6 (1–2500) × 10−6 |
Human urine | [47] |
| Dandelion pappus |
Npc/GCE | Try | 30 | (1–103) × 10−6 | Commercial amino acid injection and Fetal calf serum |
[48] |
| Bamboo | BioNC-GCE | Tmx | 220 | (0.5–35) × 10−7 | Spinach, Rice, Pear, Red soil, Tap water, Farmland water, River water, Lake water |
[49] |
| Water Hyacinth |
Biochar-rGO- CPE |
Cbz | 2.3 | (30–900) × 10−8 | Orange juice, Lettuce leaves, Drinking water, Wastewater |
[50] |
| Castor oil cake |
Ac-biochar-CPE | Caffeic acid | 30.9 | (1.0–3000) × 10−6 | White, Rose and Red wine |
[51] |
| Castor oil cake |
Ni/biochar-CPE | Glucose | 137 | (5–100) × 10−6 | Human saliva Blood serum |
[52] |
| Pumpkin stems |
Biochar-GCE | AA Dopamine UA |
2300 30 510 |
(30–95) × 10−6 (1–65) × 10−6 (2–230) × 10−6 |
SH and Human blood serum |
[53] |
| Bamboo fungus |
Biochar-GCE | Bis-A | 1068 | (0.02–10) × 10−6 | Groundwater | [54] |
| Kelp | Act-biochar-GCE | Acp | 4 | (2.5–2000) × 10−6 | Human serum And Medical tablet |
[55] |
2.2. Biochar Application in Screen-Printed-Based Platforms
| Sensor | Source | Analyte | LOD (μM) | Reference |
|---|---|---|---|---|
| nanoF-biochar-SPE | Eucalyptus scraps | o-diphenols m-phenols |
0.6 3.8 |
[68] |
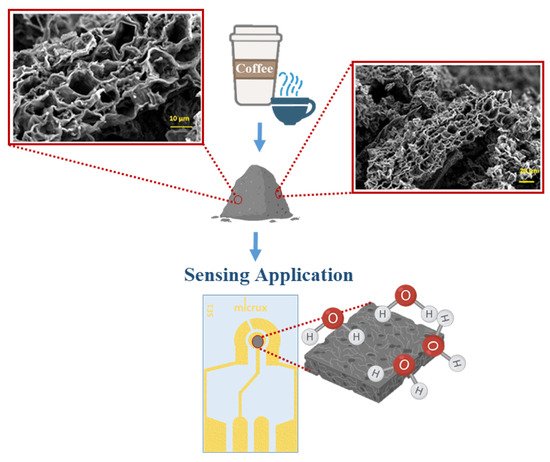
| PVB/biochar-SPE | Waste coffee ground | Humidity | 20 RH% | [69] |
| Tub-biochar-SPE | Peachwood | Lead ions | 0.02 | [70] |
| Ty-biochar-SPE | BSG | Catecholamine | 20 | [34] |
| PVP-biochar-SPE | Conifer and rapeseed pellets | Humidity | 5 RH% | [71] |
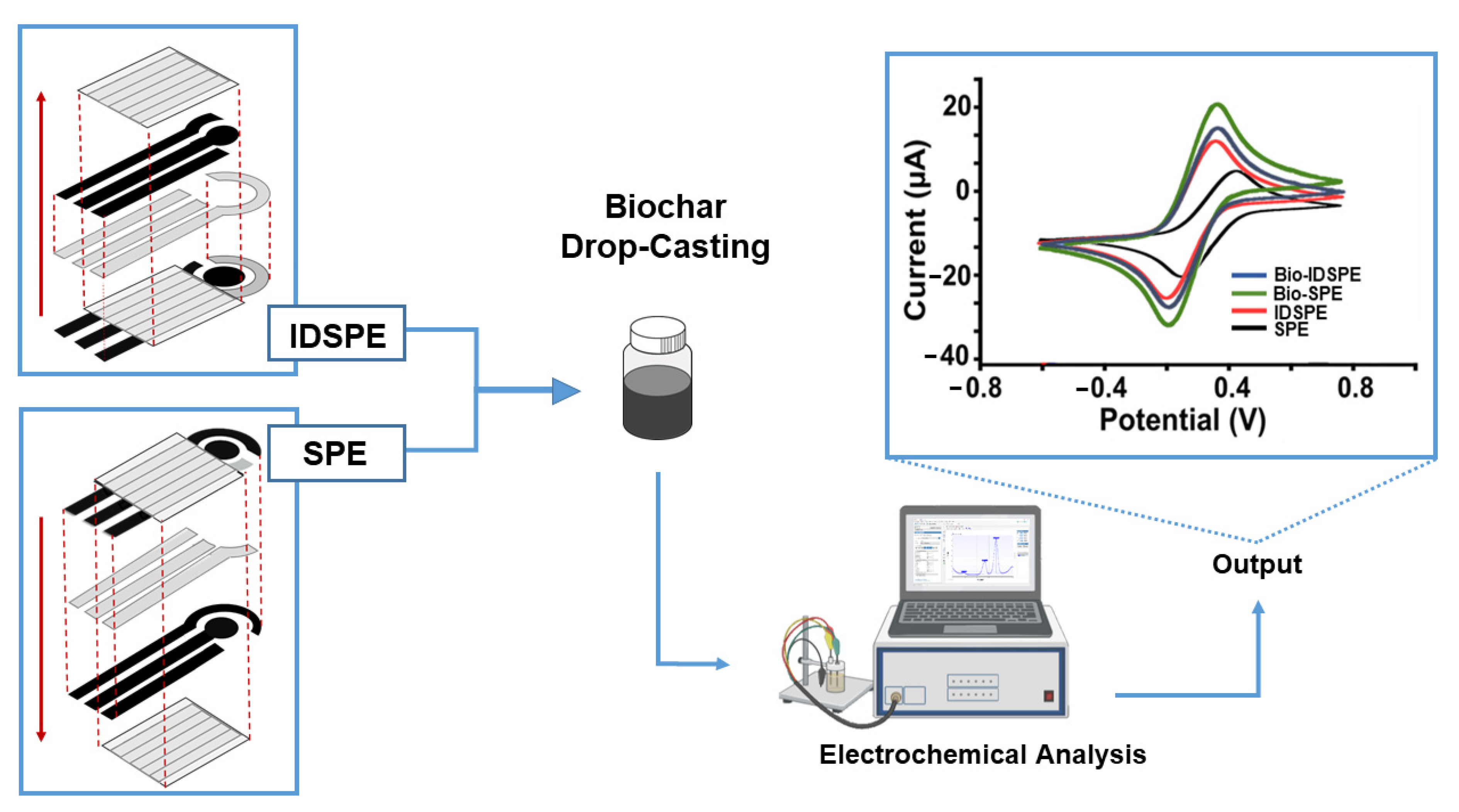
| Inverse-designed SPE | BSG | Potassium ferricyanide, AA, hexaammine ruthenium(III) chloride and NADH | 3 3 9 12 |
[72] |
| Cnf-biochar-SPE | Eucalyptus scraps | Hydroxytyrosol (o-diphenol) and tyrosol (m-phenol) | ≤0.5 ≤3.8 |
[73] |
| cMbOx-biochar-SPE | Chicken feather waste | HydroquinoneCatechol | 0.063 0.059 |
[74] |
| Laccase-carboLign-SPE | Eucalyptus globulus | Catechol | 2.01 | [75] |
| Biochar-SPE | Kudzu vine | Clenbuterol | 0.75 | [76] |
| SPE/2D activated carbon | Desmostachya bipinnata | Roxarsone | 1.5 | [77] |
| Fdop-Ac-biochar-SPE | Corchorus genus (jute) | Nitrite | 0.05 | [78] |
| Biochar-SPE | Rice husk ash | Humidity | 15% RH | [79] |
| SPE/hydrochar | Orange peels waste | Dopamine | 0.2 | [80] |
| nanoPd-Cu-Ac-biochar-SPE | Pistachio nutshells | Riboflavin | 0.0008 | [81] |
| nanoGF-Ac-biochar-nanoFeOx-SPE | Tamarind fruit shells | Rutin | 0.027 | [82] |
| Biochar-SPE | Bamboo | Humidity | 10% RH | [83] |
This entry is adapted from the peer-reviewed paper 10.3390/chemosensors10080344
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871.
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190.
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.-H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218.
- Chattopadhyay, J.; Srivastava, N. Application of Nanomaterials in Chemical Sensors and Biosensors, 1st ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-44073-2.
- Ur Rahim, H.; Qaswar, M.; Uddin, M.; Giannini, C.; Herrera, M.L.; Rea, G. Nano-Enable Materials Promoting Sustainability and Resilience in Modern Agriculture. Nanomaterials 2021, 11, 2068.
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18.
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17.
- Nsamba, H.K.; Hale, S.E.; Cornelissen, G.; Bachmann, R.T. Sustainable Technologies for Small-Scale Biochar Production—A Review. J. Sustain. Bioenergy Syst. 2015, 5, 10–31.
- Jeyasubramanian, K.; Thangagiri, B.; Sakthivel, A.; Dhaveethu Raja, J.; Seenivasan, S.; Vallinayagam, P.; Madhavan, D.; Malathi Devi, S.; Rathika, B. A complete review on biochar: Production, property, multifaceted applications, interaction mechanism and computational approach. Fuel 2021, 292, 120243.
- Cha, J.S.; Park, S.H.; Jung, S.-C.; Ryu, C.; Jeon, J.-K.; Shin, M.-C.; Park, Y.-K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15.
- Panwar, N.L.; Pawar, A.; Salvi, B.L. Comprehensive review on production and utilization of biochar. SN Appl. Sci. 2019, 1, 168.
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2010; Volume 105, pp. 47–82. ISBN 978-0-12-381023-6.
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022.
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261.
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144.
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954.
- Rahman, M.Z.; Edvinsson, T.; Kwong, P. Biochar for electrochemical applications. Curr. Opin. Green Sustain. Chem. 2020, 23, 25–30.
- Alfattani, R.; Shah, M.A.; Siddiqui, M.I.H.; Ali, M.A.; Alnaser, I.A. Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel. Energies 2021, 15, 1.
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79.
- Sun, D.; Hale, L.; Kar, G.; Soolanayakanahally, R.; Adl, S. Phosphorus recovery and reuse by pyrolysis: Applications for agriculture and environment. Chemosphere 2018, 194, 682–691.
- Inyang, M.I.; Gao, B.; Yao, Y.; Xue, Y.; Zimmerman, A.; Mosa, A.; Pullammanappallil, P.; Ok, Y.S.; Cao, X. A review of biochar as a low-cost adsorbent for aqueous heavy metal removal. Crit. Rev. Environ. Sci. Technol. 2016, 46, 406–433.
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261.
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506.
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114.
- Kane, S.; Ulrich, R.; Harrington, A.; Stadie, N.P.; Ryan, C. Physical and chemical mechanisms that influence the electrical conductivity of lignin-derived biochar. Carbon Trends 2021, 5, 100088.
- Liu, W.-J.; Jiang, H.; Yu, H.-Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285.
- Sperandio, G.; Amoriello, T.; Carbone, K.; Fedrizzi, M.; Monteleone, A.; Tarangioli, S.; Pagano, M. Increasing the value of spent grains from craft microbreweries for energy purposes. Chem. Eng. Trans. 2017, 58, 487–492.
- Joseph, S.; Husson, O.; Graber, E.; van Zwieten, L.; Taherymoosavi, S.; Thomas, T.; Nielsen, S.; Ye, J.; Pan, G.; Chia, C.; et al. The Electrochemical Properties of Biochars and How They Affect Soil Redox Properties and Processes. Agronomy 2015, 5, 322–340.
- Klüpfel, L.; Keiluweit, M.; Kleber, M.; Sander, M. Redox Properties of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2014, 48, 5601–5611.
- Zhao, L.; Cao, X.; Zheng, W.; Wang, Q.; Yang, F. Endogenous minerals have influences on surface electrochemistry and ion exchange properties of biochar. Chemosphere 2015, 136, 133–139.
- Ferreira, P.A.; Backes, R.; Martins, C.A.; de Carvalho, C.T.; da Silva, R.A.B. Biochar: A Low-cost Electrode Modifier for Electrocatalytic, Sensitive and Selective Detection of Similar Organic Compounds. Electroanalysis 2018, 30, 2233–2236.
- Kalinke, C.; Oliveira, P.R.; Oliveira, G.A.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Activated biochar: Preparation, characterization and electroanalytical application in an alternative strategy of nickel determination. Anal. Chim. Acta 2017, 983, 103–111.
- Yu, Q.; Zou, J.; Xiong, Q.; Peng, G.; Gao, F.; Fan, G.; Chen, S.; Lu, L. Electrochemical Sensor Based on Biochar Decorated with Gold Clusters for Sensitive Determination of Acetaminophen. Int. J. Electrochem. Sci. 2022, 17, 220438.
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from Brewers’ Spent Grain: A Green and Low-Cost Smart Material to Modify Screen-Printed Electrodes. Biosensors 2019, 9, 139.
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Contini, G.; Micheli, L.; Signori, E. Cost-effective and disposable label-free voltammetric immunosensor for sensitive detection of interleukin-6. Biosens. Bioelectron. 2022, 213, 114467.
- Cancelliere, R.; Di Tinno, A.; Di Lellis, A.M.; Tedeschi, Y.; Bellucci, S.; Carbone, K.; Signori, E.; Contini, G.; Micheli, L. An inverse-designed electrochemical platform for analytical applications. Electrochem. Commun. 2020, 121, 106862.
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. State-of-the-art and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301.
- Arduini, F.; Micheli, L.; Scognamiglio, V.; Mazzaracchio, V.; Moscone, D. Sustainable materials for the design of forefront printed (bio)sensors applied in agrifood sector. TrAC Trends Anal. Chem. 2020, 128, 115909.
- Suguihiro, T.M.; de Oliveira, P.R.; de Rezende, E.I.P.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. An electroanalytical approach for evaluation of biochar adsorption characteristics and its application for Lead and Cadmium determination. Bioresour. Technol. 2013, 143, 40–45.
- De Oliveira, P.R.; Lamy-Mendes, A.C.; Gogola, J.L.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. Mercury nanodroplets supported at biochar for electrochemical determination of zinc ions using a carbon paste electrode. Electrochim. Acta 2015, 151, 525–530.
- Agustini, D.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Sensitive voltammetric determination of lead released from ceramic dishes by using of bismuth nanostructures anchored on biochar. Talanta 2015, 142, 221–227.
- Oliveira, P.R.; Lamy-Mendes, A.C.; Rezende, E.I.P.; Mangrich, A.S.; Marcolino Junior, L.H.; Bergamini, M.F. Electrochemical determination of copper ions in spirit drinks using carbon paste electrode modified with biochar. Food Chem. 2015, 171, 426–431.
- Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Carbon Paste Electrode Modified with Biochar for Sensitive Electrochemical Determination of Paraquat. Electroanalysis 2016, 28, 764–769.
- Oliveira, P.R.; Kalinke, C.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Copper hexacyanoferrate nanoparticles supported on biochar for amperometric determination of isoniazid. Electrochim. Acta 2018, 285, 373–380.
- De Oliveira, P.R.; Kalinke, C.; Gogola, J.L.; Mangrich, A.S.; Junior, L.H.M.; Bergamini, M.F. The use of activated biochar for development of a sensitive electrochemical sensor for determination of methyl parathion. J. Electroanal. Chem. 2017, 799, 602–608.
- Dong, X.; He, L.; Liu, Y.; Piao, Y. Preparation of highly conductive biochar nanoparticles for rapid and sensitive detection of 17β-estradiol in water. Electrochim. Acta 2018, 292, 55–62.
- Zhang, W.; Liu, L.; Li, Y.; Wang, D.; Ma, H.; Ren, H.; Shi, Y.; Han, Y.; Ye, B.-C. Electrochemical sensing platform based on the biomass-derived microporous carbons for simultaneous determination of ascorbic acid, dopamine, and uric acid. Biosens. Bioelectron. 2018, 121, 96–103.
- Han, J.; Zhao, J.; Li, Z.; Zhang, H.; Yan, Y.; Cao, D.; Wang, G. Nanoporous carbon derived from dandelion pappus as an enhanced electrode material with low cost for amperometric detection of tryptophan. J. Electroanal. Chem. 2018, 818, 149–156.
- Chen, S.; Li, L.; Wen, Y.; Yang, G.; Liu, G.; Yi, Y.; Shang, Q.; Yang, X.; Cai, S. Voltammetric Analysis of Thiamethoxam Based on Inexpensive Thick-Walled Moso Bamboo Biochar Nanocomposites. Int. J. Electrochem. Sci. 2019, 14, 10848–10861.
- Sant’Anna, M.V.S.; Carvalho, S.W.M.M.; Gevaerd, A.; Silva, J.O.S.; Santos, E.; Carregosa, I.S.C.; Wisniewski, A.; Marcolino-Junior, L.H.; Bergamini, M.F.; Sussuchi, E.M. Electrochemical sensor based on biochar and reduced graphene oxide nanocomposite for carbendazim determination. Talanta 2020, 220, 121334.
- Kalinke, C.; Zanicoski-Moscardi, A.P.; de Oliveira, P.R.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Simple and low-cost sensor based on activated biochar for the stripping voltammetric detection of caffeic acid. Microchem. J. 2020, 159, 105380.
- Kalinke, C.; Wosgrau, V.; Oliveira, P.R.; Oliveira, G.A.; Martins, G.; Mangrich, A.S.; Bergamini, M.F.; Marcolino-Junior, L.H. Green method for glucose determination using microfluidic device with a non-enzymatic sensor based on nickel oxyhydroxide supported at activated biochar. Talanta 2019, 200, 518–525.
- Ramalechume, C.; Mageswari, G.; Swamidoss, C.M.A. Determination of dopamine in the presence of ascorbic acid using polyaniline/carbon dot composites. Mater. Today Proc. 2020.
- Liu, Y.; Yao, L.; He, L.; Liu, N.; Piao, Y. Electrochemical Enzyme Biosensor Bearing Biochar Nanoparticle as Signal Enhancer for Bisphenol A Detection in Water. Sensors 2019, 19, 1619.
- Shan, R.; Shi, Y.; Gu, J.; Wang, Y.; Yuan, H. Single and competitive adsorption affinity of heavy metals toward peanut shell-derived biochar and its mechanisms in aqueous systems. Chin. J. Chem. Eng. 2020, 28, 1375–1383.
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35.
- Li, Y.; Shao, J.; Wang, X.; Deng, Y.; Yang, H.; Chen, H. Characterization of Modified Biochars Derived from Bamboo Pyrolysis and Their Utilization for Target Component (Furfural) Adsorption. Energy Fuels 2014, 28, 5119–5127.
- Oo, A.Z.; Sudo, S.; Akiyama, H.; Win, K.T.; Shibata, A.; Yamamoto, A.; Sano, T.; Hirono, Y. Effect of dolomite and biochar addition on N2O and CO2 emissions from acidic tea field soil. PLoS ONE 2018, 13, e0192235.
- He, L.; Yang, Y.; Kim, J.; Yao, L.; Dong, X.; Li, T.; Piao, Y. Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol A sensing in water. Chem. Eng. J. 2020, 384, 123276.
- Sobhan, A.; Muthukumarappan, K.; Wei, L.; Qiao, Q.; Rahman, M.T.; Ghimire, N. Development and characterization of a novel activated biochar-based polymer composite for biosensors. Int. J. Polym. Anal. Charact. 2021, 26, 544–560.
- Zhou, Q.; Guo, M.; Wu, S.; Fornara, D.; Sarkar, B.; Sun, L.; Wang, H. Electrochemical sensor based on corncob biochar layer supported chitosan-MIPs for determination of dibutyl phthalate (DBP). J. Electroanal. Chem. 2021, 897, 115549.
- Kalinke, C.; Oliveira, P.R.; Bonet San Emeterio, M.; González-Calabuig, A.; Valle, M.; Salvio Mangrich, A.; Humberto Marcolino Junior, L.; Bergamini, M.F. Voltammetric Electronic Tongue Based on Carbon Paste Electrodes Modified with Biochar for Phenolic Compounds Stripping Detection. Electroanalysis 2019, 31, 2238–2245.
- Martins, G.; Gogola, J.L.; Caetano, F.R.; Kalinke, C.; Jorge, T.R.; Santos, C.N.D.; Bergamini, M.F.; Marcolino-Junior, L.H. Quick electrochemical immunoassay for hantavirus detection based on biochar platform. Talanta 2019, 204, 163–171.
- Kerr, E.; Alexander, R.; Francis, P.S.; Guijt, R.M.; Barbante, G.J.; Doeven, E.H. A Comparison of Commercially Available Screen-Printed Electrodes for Electrogenerated Chemiluminescence Applications. Front. Chem. 2021, 8, 628483.
- Cancelliere, R.; Tinno, A.D.; Cataldo, A.; Bellucci, S.; Micheli, L. Powerful Electron-Transfer Screen-Printed Platforms as Biosensing Tools: The Case of Uric Acid Biosensor. Biosensors 2021, 12, 2.
- Di Tinno, A.; Cancelliere, R.; Mantegazza, P.; Cataldo, A.; Paddubskaya, A.; Ferrigno, L.; Kuzhir, P.; Maksimenko, S.; Shuba, M.; Maffucci, A.; et al. Sensitive Detection of Industrial Pollutants Using Modified Electrochemical Platforms. Nanomaterials 2022, 12, 1779.
- Sasirekha, N.; Chen, Y.W. Carbonaceous Nanomaterials for Environmental Remediation; Springer: Cham, Switzerland, 2021.
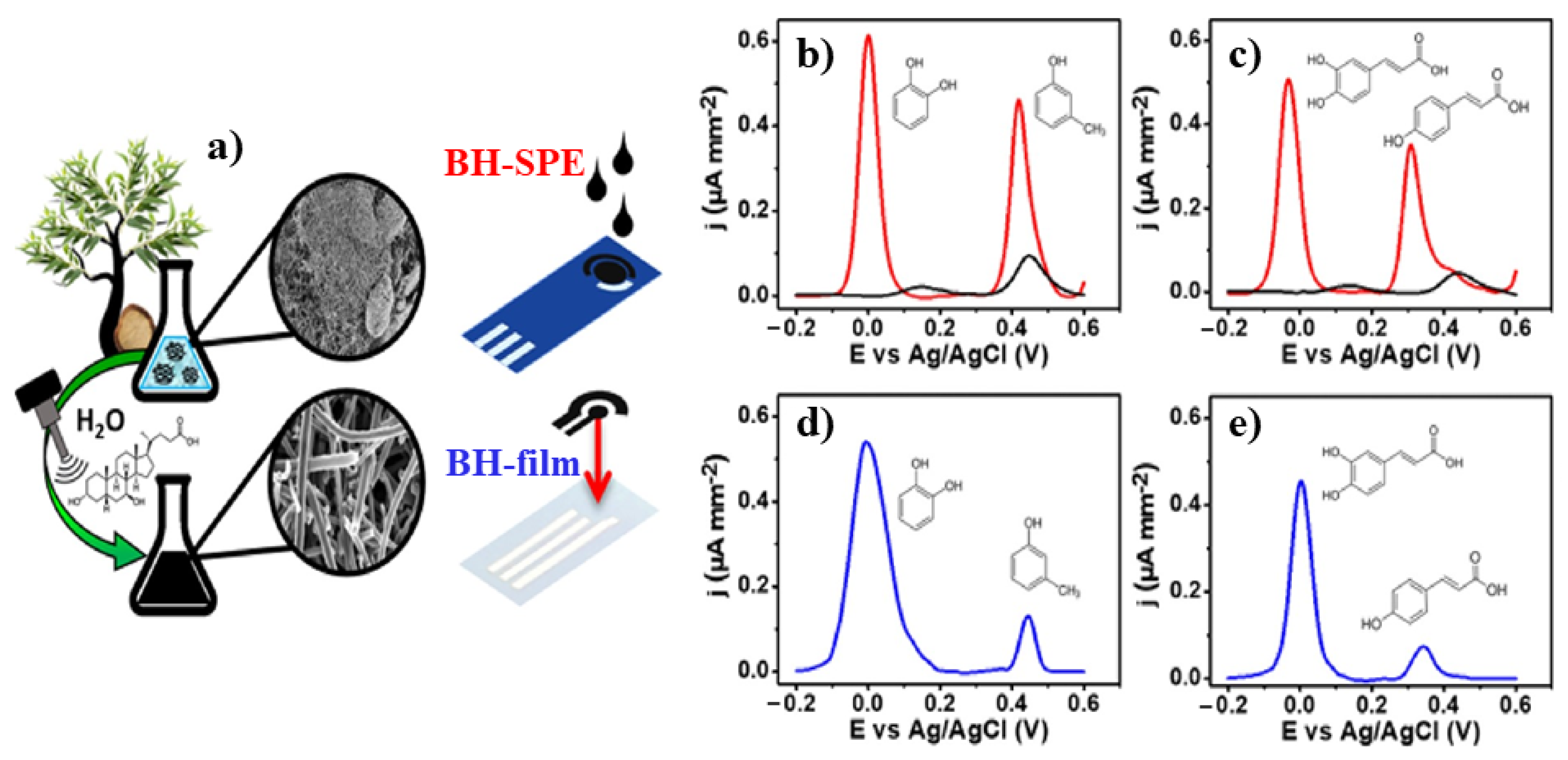
- Bukhari, Q.U.A.; Silveri, F.; Della Pelle, F.; Scroccarello, A.; Zappi, D.; Cozzoni, E.; Compagnone, D. Water-Phase Exfoliated Biochar Nanofibers from Eucalyptus Scraps for Electrode Modification and Conductive Film Fabrication. ACS Sustain. Chem. Eng. 2021, 9, 13988–13998.
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.; Tagliaferro, A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801.
- Chen, X.; Lu, K.; Lin, D.; Li, Y.; Yin, S.; Zhang, Z.; Tang, M.; Chen, G. Hierarchical Porous Tubular Biochar Based Sensor for Detection of Trace Lead (II). Electroanalysis 2021, 33, 473–482.
- Ziegler, D.; Palmero, P.; Giorcelli, M.; Tagliaferro, A.; Tulliani, J.-M. Biochars as Innovative Humidity Sensing Materials. Chemosensors 2017, 5, 35.
- Scroccarello, A.; Pelle, F.D.; Bukhari, Q.U.A.; Silveri, F.; Zappi, D.; Cozzoni, E.; Compagnone, D. Eucalyptus Biochar as a Sustainable Nanomaterial for Electrochemical Sensors. Chem. Proc. 2021, 5, 13.
- Ganesan, S.; Sivam, S.; Elancheziyan, M.; Senthilkumar, S.; Ramakrishan, S.G.; Soundappan, T.; Ponnusamy, V.K. Novel delipidated chicken feather waste-derived carbon-based molybdenum oxide nanocomposite as efficient electrocatalyst for rapid detection of hydroquinone and catechol in environmental waters. Environ. Pollut. 2022, 293, 118556.
- Zappi, D.; Varani, G.; Cozzoni, E.; Iatsunskyi, I.; Laschi, S.; Giardi, M.T. Innovative Eco-Friendly Conductive Ink Based on Carbonized Lignin for the Production of Flexible and Stretchable Bio-Sensors. Nanomaterials 2021, 11, 3428.
- Zhang, K.; Ge, Y.; He, S.; Ge, F.; Huang, Q.; Huang, Z.; Wang, X.; Wen, Y.; Wang, B. Development of New Electrochemical Sensor based on Kudzu Vine Biochar Modified Flexible Carbon Electrode for Portable Wireless Intelligent Analysis of Clenbuterol. Int. J. Electrochem. Sci. 2020, 15, 7326–7336.
- Nikhil; Srivastava, S.K.; Srivastava, A.; Srivastava, M.; Prakash, R. Electrochemical Sensing of Roxarsone on Natural Biomass-Derived Two-Dimensional Carbon Material as Promising Electrode Material. ACS Omega 2022, 7, 2908–2917.
- Ahammad, A.J.S.; Pal, P.R.; Shah, S.S.; Islam, T.; Hasan, M.; Qasem, M.A.A.; Odhikari, N.; Sarker, S.; Kim, D.M.; Aziz, A. Activated jute carbon paste screen-printed FTO electrodes for nonenzymatic amperometric determination of nitrite. J. Electroanal. Chem. 2019, 832, 368–379.
- Ziegler, D.; Boschetto, F.; Marin, E.; Palmero, P.; Pezzotti, G.; Tulliani, J.-M. Rice husk ash as a new humidity sensing material and its aging behavior. Sens. Actuators B Chem. 2021, 328, 129049.
- Espro, C.; Satira, A.; Mauriello, F.; Anajafi, Z.; Moulaee, K.; Iannazzo, D.; Neri, G. Orange peels-derived hydrochar for chemical sensing applications. Sens. Actuators B Chem. 2021, 341, 130016.
- Sangili, A.; Veerakumar, P.; Chen, S.-M.; Rajkumar, C.; Lin, K.-C. Voltammetric determination of vitamin B2 by using a highly porous carbon electrode modified with palladium-copper nanoparticles. Microchim. Acta 2019, 186, 299.
- Elancheziyan, M.; Ganesan, S.; Theyagarajan, K.; Duraisamy, M.; Thenmozhi, K.; Weng, C.-H.; Lin, Y.-T.; Ponnusamy, V.K. Novel biomass-derived porous-graphitic carbon coated iron oxide nanocomposite as an efficient electrocatalyst for the sensitive detection of rutin (vitamin P) in food and environmental samples. Environ. Res. 2022, 211, 113012.
- Mahmoud, M.E.; Khalifa, M.A.; El Wakeel, Y.M.; Header, M.S.; El-Sharkawy, R.M.; Kumar, S.; Abdel-Fattah, T.M. A novel nanocomposite of Liquidambar styraciflua fruit biochar-crosslinked-nanosilica for uranyl removal from water. Bioresour Technol. 2019, 278, 124.
- Afify, A.S.; Ahmad, S.; Khushnood, R.A.; Jagdale, P.; Tulliani, J.-M. Elaboration and characterization of novel humidity sensor based on micro-carbonized bamboo particles. Sens. Actuators B Chem. 2017, 239, 1251–1256.
