Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Vitis vinifera, known as the common grape vine, represents one of the most important fruit crops in the world. Romania is a wine-producing country with a rich and long tradition in viticulture. Increasing reports of damage caused by grapevine trunk diseases (GTDs) have raised concerns in all wine producing countries.
- fungal pathogens
- grapevine trunk diseases (GTDs)
- Romanian vineyards
1. Eutypa Dieback in Romania
In Romania, Eutypa lata was first mentioned by Rafailă and Oprea as found in the vineyards of Comarna, Cotnari, and Bucium, in Iași County (VZ2), in 1982 and later in other vineyards all around the country: Miniș (VZ5), Diosig (VZ5), Jidvei (VZ1), Dealul Mare (VZ3), Valea Călugărească (VZ3), Odobești (VZ2), Murfatlar (VZ6), Ostrov (VZ7) [1][2], Pietroasele (VZ8), Alba (VZ1), and Apold (VZ1) [2][3][4][5][6] (Figure 1).

Figure 1. Graphical representation of Eutypa dieback reports in Romania.
1.1. Risk Factors
Eutypa lata infection associated with ecological weakening factors determined the grapevine’s dieback all over Romania’s vineyards [7]. The percentage of infested grapevine trunks and canes was between 3% and 96%; the disease was found in vineyards older than 6 years of age, especially on the ones of 10–15 years old [7]. Thus, the age of the plantation has a significant influence on the presence of Eutypa dieback [4]. This does not necessarily imply that young plantations are less vulnerable to Eutypa lata infection; the absence of symptoms is attributable to the pathogen’s delayed evolution in grapevine wood [4]. The disease is found with a much higher frequency in neglected vineyards, where the phytosanitary measures were not applied according to the recommendations of the specialists [8].
No resistant cultivars to the attack of Eutypa lata fungus are known [9][10]. Oprea and Podosu [7] present in their study the most sensitive grapevine cultivars as being the ones for wine, especially Italian riesling (in Diosig—Bihor and Gaiceanca—Bacău vineyards) where on certain plots, all vine trunks and canes showed specific symptoms of Eutypa lata infection [7]. Other cultivars very much affected by the disease were: 60% Cabernet Sauvignon, over 30% Fetească albă, between 10 and 89% Băbească neagră (much severe in Nicoreşti—Galaţi vineyard), about 63% Fetească regală, 30% Muscat Ottonel (especially in Miniș vineyard), and between 3 and 33% of the autochthonous cultivars: Grasă de Cotnari, Galbenă de Odobești, and Șarbă [7]. The most infected grapevine cultivars for table grapes were Chasselas d’Oré (68% in Valea Călugărească vineyard) and Afuz Ali (in south Romania vineyards) [2][7][10].
In Târnave vineyards Fetească regală, Fetească albă, Sauvignon blanc, and Muscat Ottonel cultivars were assessed for the Eutypa dieback infection evolution in the vine plantations of different ages: young vineyards of 6 years and old vineyards over 25 years [4]. The attack degree (AD) was found to be substantially lower (1.5%) in young plantations compared to older ones (18%), regardless of the cultivar [4]. In terms of studied cultivar, independently of the plantation age, Feteasca regală (7.0%) and Sauvignon blanc (7.3%) had the lowest AD, followed by Feteasca albă (8.7%) and Muscat Ottonel (15%) as the most sensitive to Eutypa lata in the microclimate of Târnave vineyard.
1.2. Symptoms
Overall, the Eutypa lata fungus produces metabolic disorders in the vine, and the characteristic symptoms of Eutypa dieback appear under the influence of the released toxins. The winter climatic conditions (low temperatures and precipitation quantities) may influence the characteristic symptoms expression. When the disease has a powerful expression of symptoms, during the start of the vegetation period of the grapevine, a proliferation of shoots is observed, and they continue to have an abnormal development for the rest of the vine’s life [2]. The leaves remain tiny, chlorotic, and distorted with ragged borders [1][2][10][11]. The inflorescences may wither after flowering or form clusters of beaded berries [1][2]. The first-year canes do not develop normally; they remain short, thin, and with an obvious short knotting. Oprea and Dumitru [2] state in their work that the normal autumn canes measure 93.8 cm on average, compared to the annual canes of the vines affected by Eutypa dieback that measured between 19.55 and 22.3 cm. In autumn, the fruit clusters on the affected vines remain very small. Oprea and Dumitru [2] show an average weight of the fruit cluster of 5.8–12.8 g on an affected vine compared to 72 g of a healthy, normal fruit cluster. In sections, the affected arm presents specific wedge-shaped necrosis, in the xylem area, where the primary infections took place. Phloem and cortical tissue, secondarily invaded, form ulcerative lesions. In a more advanced stage of the disease, the fungus invades and destroys the secondary walls of the libero-ligneous vessel; the tissue becomes very frail, and thus, the wood can easily break on bending [1][2][7]. Cross sections of the affected wood present distinct, hard V-shaped necrosis, with the margins of contaminated areas turning brown-gray to brown-red/purple depending on variety [7][11].
1.3. Fungi Involved
Eutypa dieback is propagated, from year to year, mostly by Eutypa lata ascospores that grow in the perithecia of dry wood. They can stay viable for up to five years, which is why Eutypa-infected stocks can be a long-term source of infection [1][2][11]. Infections develop in late autumn and early spring, when rainfall is favorable [11]. Although Eutypa lata is the well-known and studied fungal pathogen inducing Eutypa dieback, in the Blaj-Târnave vineyard, on Victoria grapevines, other Diatrypaceae sp. were also identified on trunk samples infected with Eutypa dieback GTD [5].
Biological parameters such as temperature, pH values, and humidity, as well as the energy sources, such as carbon and light, may have an influence on the development of pathogenic fungi. On this matter, Oprea and Podosu [7] conducted a study in order to determine the influence of the biological parameters on the development of Eutypa lata colonies.
Temperature Influence on the In Vitro Development of Eutypa lata
The development of Eutypa lata colonies on Chloramphenicol Glucose Agar (CGA) medium was strongly influenced by the temperature levels. The fungi colonies start to grow at +8 °C, and they show a white, lax mycelium which is observed as yellow on reverse [5][7]. After 15 days, the colonies reach 20 mm diameter. In these conditions, fructifications were not formed. As the temperature rises, the colonies diameter increases, the mycelium becomes dense and takes a felt aspect. The optimal temperature for E. lata’s development was registered between 18 and 26 °C. Black crusts developed on the mycelium surface, and gray pycnidia appeared due to the abundance of hyphae. The maturation of pycnidia takes 12–14 days and is contained in a gelid mass that is eliminated via ostiols. Perithecia continued to be formed in several of the cultures incubated at 18 °C [7].
The highest temperature for Eutypa lata’s development was found to be 34 °C, and 36 °C may be considered the lethal level for this fungus. When the temperature dropped from 36 °C to 22 °C, the colonies on Petri plates continued to produce mycelium. The temperature also has an important role in determining the germination of Eutypa lata spores. Pycniospores do not germinate on the medium culture (water, agar, or maltose 5%) in the amorphous stage unless they are not exposed to UV rays for 15 min. Germination began two hours after irradiation at a low percentage (2%), and the length of the germination tube was 1.5–2 µm. The pycniospores germination percentage grew with the duration of the irradiation exposure time, and as a consequence, after 24 h, spore germination was 46%, and germination tube length was 52–65 µm. In the typical conditions of water–agar medium, ascospores present in the teleomorph stage germinated. Germination began at 10 °C and continued until 30°C; the ideal temperature was between 18 and 28 °C [7].
Relative Atmospheric Humidity (RH) Influence on the In Vitro Development of Eutypa lata
Besides temperature, the development of Eutypa lata fungal colonies is controlled by relative air humidity levels, according to observations made in vitro. Mycelium development was halted by humidity levels below 30.5%, but it became abundant without fructifications at RH values between 39% and 74%. When the humidity level exceeds 80%, morphological changes occur. The fungus fructified and produced pycnidia after 4 weeks that were matured after 7–11 days [7].
Influence of pH Values on the In Vitro Development of Eutypa lata
Eutypa lata, the observed lignicoulus fungus, grew in an acidic (4) to basic (11) pH growth media. The fungus displayed limited development colonies with a low vegetative mass at the acid level (4). Fungi colonies with a rich vegetative mass and pycnidial fructifications were formed from low acid to strong basic pH (5.5–11) [7].
The Energetic Resources of Eutypa lata Growing In Vitro
- (a)
-
Carbon source influence
The findings of the in vitro experiments revealed that carbon was the most critical factor for the formation of the lignicoulus fungus colonies. This element, a component of the carbohydrate molecules, can be assimilated in various ways depending on the source and the carbon chemical link in that molecule. Carbon is hydrolyzed from monosaccharides such as glucose, fructose, trehalose, ribose, arabinose, levulose, and rhamnose. Melibiose and sucrose are the hydrolyzed disaccharides, and cellulose and lignin are the polysaccharides ones. The colonies developed on a growth medium which included these carbon resources, grew to their fullest size, and fructified well. On sorbosis growth medium, as well as on galactosis, maltosis, and inulinase media, Eutypa lata developed more slowly. The fungus thrives on manitosis, galactosis, levulosis, melibiosis, and starch culture medium; however, it does not fructify. Colonies of Eutypa lata fungi were unable to grow in the absence of carbon [7].
- (b)
-
Light influence
The CGA growth medium on Petri plates containing fungal colonies was exposed to a constant source of light, light/dark alternating (8 h with 16 h or 12 h with 12 h), and continuous dark. The fungus colonies that were kept in a constant dark environment had the best vegetative mass; the colonies had a dense, felt-like appearance. However, weak fructifications were developed. In a light dark alternation of 8 to 16 h, Eutypa lata fructified very effectively [7].
2. Phomopsis Dieback in Romania
In Romania, the first report of Phomopsis dieback (well known as excoriosis) was made by Săvulescu et al., in “Starea fitosanitară” [Phytosanitary Status -RO] in 1926–1929 and 1932–1933, but they attributed the disease to other pathogen agents (Phoma flaccida, Phoma reniformis). This confusion has lasted for some time, as it did in the rest of the world, many authors reporting excoriosis symptoms attributed to other fungal species. [1][10]. Phomopsis viticola, the etiological agent of grapevine excoriosis, was first identified in Romania in 1962 by Crișan. In 1970, Rafailă reported the GTD in various Romanian vineyards describing characteristic symptoms and connecting for the first time the excoriosis with Phomopsis viticola Sacc. Due to the expansion of the affected area and the damage caused, especially after 1967–1968, many Romanian researchers, such as Oprea and Dumitru [2], Mărmureanu [12], Tică [13][14], and Ulea [1][15], have studied excoriosis. All authors highlight the practical importance of this GTD, which causes significant losses in some of the Romanian vineyards, such as Iași (VZ2), Cotnari (VZ2), Dealul-Bujorului (VZ2), Odobești (VZ2), Murfatlar (VZ6), Jidvei (VZ1), Alba (VZ1), Ciumbrud-Aiud (VZ1), Miniș (VZ5), Arad (VZ5), Dragășani (VZ3), Bihor (VZ5), Valea Călugărească (VZ3), Pietroasele (VZ3), Panciu (VZ2), and Ostrov (VZ7) [1][4][5][6][14][16][17] (Figure 2).
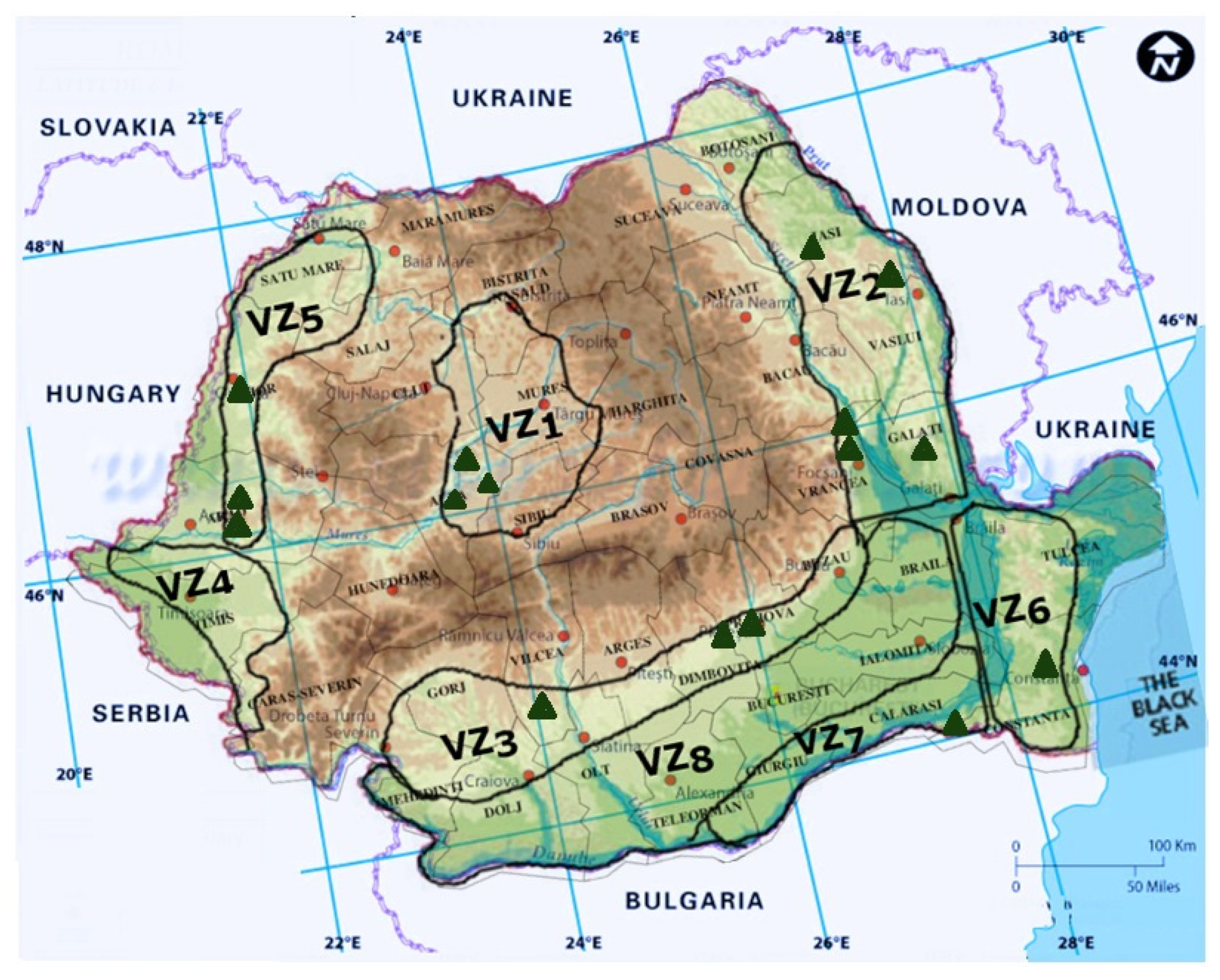
Figure 2. Graphical representation of Phomopsis dieback reports in Romania.
Ulea [1] reports excoriosis in Moldova’s vineyards during 1968–1992 as follows: in 1982 in Cotnari and Iași vineyards (VZ2) at a 0.01% attack degree (AD), in 1983 in Iași and Odobești vineyards at 0.25% AD, again in 1985, in Odobești vineyard at 0.25% AD, in 1990 in Iași vineyard at 1.5% AD, also in 1991 in Iași and Odobești vineyards at 3% AD and in 1992 in Iași vineyard at 0.01% AD [1]. In the Odobești vineyard, the following cultivars were detected with this GTD: Fetească regală, Italian Riesling, Șarbă, Furmint, Chasselas d’Oré, Galbenă de Odobești, and Plăvaie. Feteasca regală, Italian Riesling, Șarba, and Chasselas d’Oré cultivars had excoriosis also in the Panciu vineyard, and besides these, the cultivars Rkatiteli and Aligote. In the Cotești vineyard, Phomopsis viticola infected Fetească regală, Italian Riesling, Șarbă, Chasselas d’Oré, Aligote, Plăvaie, Fetească albă, Hamburg Muscat, and Afuz-Ali cultivars [17]. In the Târnave vineyard (VZ1) the Phomopsis viticola’s AD was evaluated in May, and this parameter was 28.06% for Fetească regală, 27.86% for Italian Riesling, 22.50% for Sauvignon blanc, and much lower (of 5.70%) for Traminer roz [18]. Phomopsis viticola was found to cause excoriosis on the Traminer cultivar also in the Aiud-Ciumbrud vineyard (VZ1) [5].
According to Oprea and Podosu [7], grapevines growing in places with a clay compact acid soil, excessive watering, and industrial pollution are more impacted by excoriosis [7]. In Vrancea vineyards (VZ2), the loss rate in vineyards located in river meadows, on low, cold, and humid terrains, reached 35% [17]. It was also reported that the behavior of grapevine cultivars in response to this disease under natural infection conditions varies depending on the pedological and climatic characteristics of the vineyard. However, due to their genetics, some cultivars like Fetească regală, Sauvignon blanc, and Italian Riesling are more susceptible to this fungal disease than others [18].
Tică et al. [13][14], by comparing the behavior of the grapevine cultivars to the attack of Phomopsis viticola in the conditions of Vrancea vineyards (VZ2) (Odobești, Cotești, and Panciu), conclude that in conditions of natural infection, the susceptibility of the cultivars may differ for the same cultivar depending on the pedoclimatic and microclimatic conditions of the vineyard. Thus, the Plăvaie cultivar oscillated between very sensitive and sensitive (AD = 16.6–52.4%), the cultivars Galbenă de Odobești and Furmint between medium-resistant and sensitive (AD = 6.6–17.3%), and the cultivars Fetească regală, Fetească albă, Italian Riesling, and Șarbă between resistant and sensitive (AD = 1.3–14.2%). The Afuz-Ali cultivar behaved as sensitive (AD = 12.3–22.2%). The cultivars Merlot, Frâncușă, Chasselas d’Oré, Aligote (AD = 0.1–1.3%), and Muscat de Hamburg (AD = 1.5–2.2%) proved to be tolerant [14][17]. From the data obtained in the experiment but also from the data in the specialized literature, the Merlot cultivar showed tolerance in Vrancea, in the Dealul Mare vineyard, and in those in France, while the Aligote and Chasselas d’Oré cultivars, which became tolerant in Romania, are sensitive to the pathogen in France [17].
Oprea and Dumitru [2] followed the behavior of grapevine cultivars when attacked by Phomopsis viticola in Valea Călugărească (VZ3), and they found that Cardinal, Cabernet Sauvignon, Fetească regală, Muscat de Hamburg, Muscat Ottonel, Mustoasă, and Clairete are very sensitive (AD = 40.31–54.68%). The plots in which the phenomenon of vines drying was more severe were located on flat ground, with soil that maintained humidity [2].
2.1. Symptoms
In Romania, the symptoms of excoriosis were discussed in the complex of premature death of grapevines by Oprea and Dumitru [2] and Mărmureanu et al. [12], and they consider that excoriosis is a focal point disease. Its spread is primarily due to human intervention, grafting being the main cause, as a result of the use of infected canes. The damages thus appear from the nursery [2]. In Romania, in the period 1986–1990, Tică et al. [13] investigated the influence of the pathogen Phomopsis viticola, present in grafting eyes, on the phytosanitary condition of the vine during the grafting–forcing period and on vines planted in the nursery, in Odobești vineyards. From the data analysis, it was established that the yield of STAS vines decreased in the excoriosis attacked vines group from 31.5% to 15.7% [1][13].
Another aspect studied by the same authors was the influence of excoriosis on budburst. Several cultivars were studied, and the conclusion is that the buds at the base of the shoots are more affected than those located at the top of the shoots—13–81% buds from the 1st to 4th eye that did not start vegetation in spring compared to 6–18% of the buds located at the upper part (7–10th eye). The best start in vegetation was recorded in the last eyes on the shoots (96–98%) [1][13].
Same authors also studied the influence of excoriosis on grape production of various cultivars in terms of quantity and quality. The results show that grape production decreases in the case of infected vines with up to 48.4% (Plăvaie cultivar). The lowest drop of grape production was 5.9% registered on the Chasselas d’Oré cultivar. Galbenă de Odobești, Furmint, Italian Riesling, and Șarbă registered decreases of grape production ranging between 39.7% and 27.2%. These differences were linked to the attack degree and thus to the cultivars’ disease tolerance [1][13]. Podosu et al. [17] also established that during the offshoot growth phase, damage is significant, and the influence of this damage on the quantity and quality of the yield has been assessed.
The sugar content of grapes from infected vines was lower compared to the healthy ones (an average of 165.5 g/L compared to an average of 174.4 g/L). The acidity of the must (grape juice) was not influenced by excoriosis, pH values of the grape juices not showing significant differences [1][14].
The first symptoms of excoriosis appear at the beginning of the vegetation period, when bud break is delayed, under the form of dark spots isolated or associated of about 0.5–2 cm × 0.3–1.0 cm, usually located at the base of canes [11][13][17]. In the Valea Călugărească vineyard, the budburst period was 12–14 days delayed, and the basal buds were dead [17]. As the infection evolves, ulcerations occur, budding is delayed, and the buds at the base of the canes die, leaving only those at the top viable, which pushes the fruit elements higher on the shoots. Black, small, round, or linear, more or less deep lesions appear in the shoots. In Târnave vineyard when the observations were done later (May–July), cane and leaf spots were observed [18]. On the leaves, petioles, and then on racemes and pedicels, during summer, appear circular spots, brown-blackish in the center with a yellow-orange halo. During strong attacks, the shoots remain small, stunted, sometimes in a fan form, and they detach easily. After entering the veraison, the grape berries rot and are, sometimes, covered with the characteristic pycnidia [1][11][14]. In the fall, on the canes, Ulea [1] signaled a pronounced whitening of the bark, especially on the first basal internodes, on which small black pycnidia are defined. At the end, the vine dies in the summer, with the leaves and fruit clusters on it [1][11][14]. The infections are favored by cold and wet weather [11]. For instance, in the Târnave vineyard for the period 2009–2011, Phomopsis viticola had a higher intensity in the vineyard in 2010, the wetter and colder year of the interval. This allowed the authors to establish correlations between disease intensity and weather conditions [11].
2.2. Fungi Involved
Life Cycle of Phomopsis viticola
The Phomopsis viticola Sacc. fungus survives the winter under pycnids form on the basal arms and as mycelium in the dormant buds [13][17][19]. During this time, the pycnids formed in autumn mature. The pycniospores (α and β of which α spores are the most important source of infection) start to form near the end of winter (in February) and are present in large quantities in spring in the sprouting phenophase [2][13][17] (Figure 3).
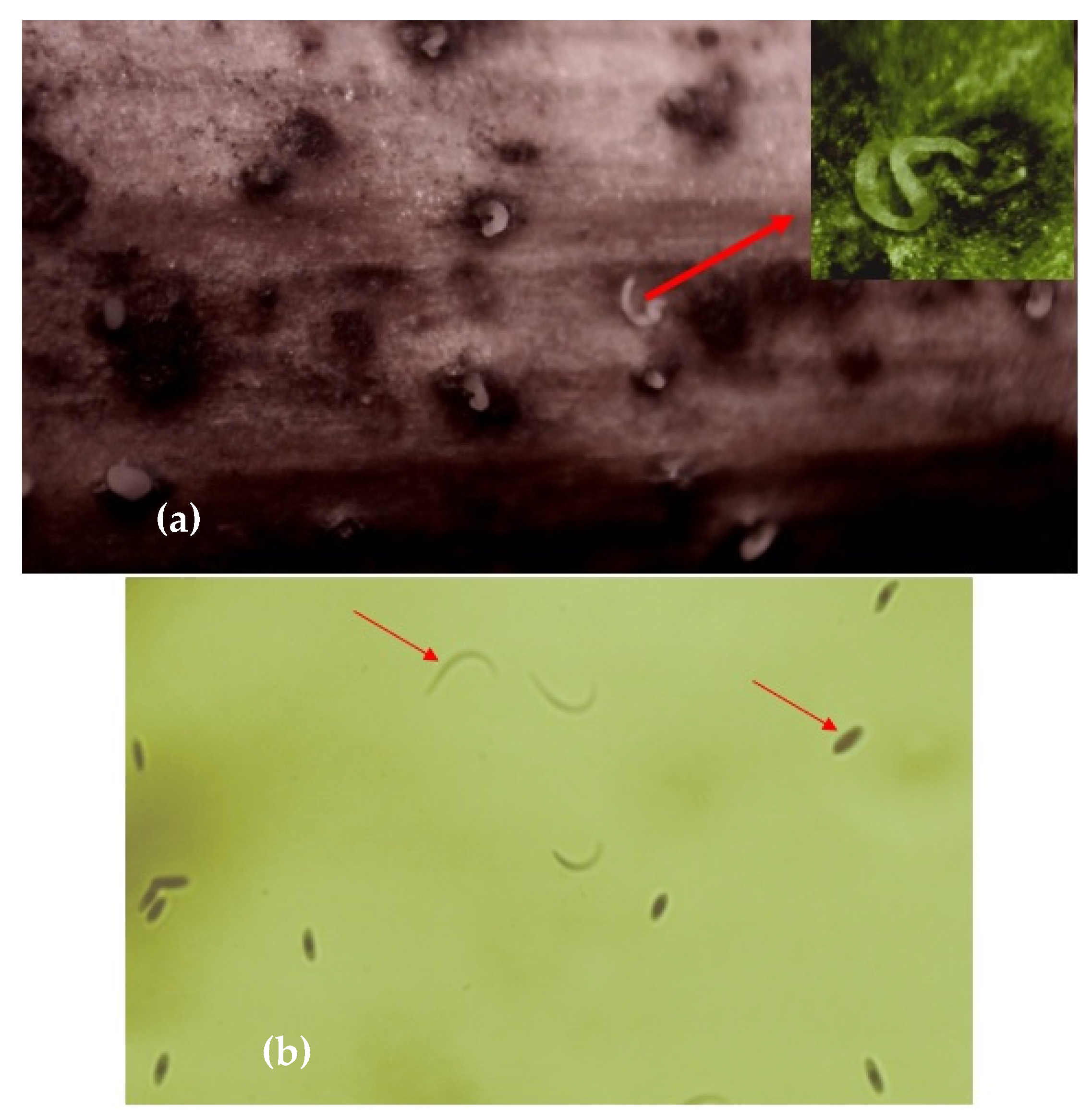
Figure 3. Pycnidia (a) and pycniospores (b) (α and β) of Phomopsis viticola.
Mature conidia (pycniospores) are dispersed by rain drops, and they germinate on the canes in 4 to 8 h depending on the temperatures (25 °C optimal temperature; −15 °C lower limit). After pycnospore germinations, the infection starts in 5 h in optimal temperature conditions (25 °C) [13]. At a temperature range of 15–18°C, the infection begins after 7 to 10 h of continuous humidity of the vine [13][17][20]. The most sensitive to infections are the young offshoots, from the beginning of the shoot growth until the spring rains [17].
Biology of Phomopsis viticola
Podosu et al. [17] isolated the pathogen Phomopsis viticola Sacc. from samples consisting in grapevine arms, shoots and roots, withered or dry, collected from Vrancea’s vineyards, which showed signs of decline. The samples were kept in the humid chamber at temperatures of 18–22 °C. After fructification, the fungus was isolated on a GCA medium and then purified in test tubes or Petri dishes. The in vitro biological aspects sought to determine the influence of some agarized culture media of the GCA culture medium reactions and the effect of temperature and relative humidity on the growth and spore-production of the fungus.
The Phomopsis viticola Sacc. isolation was obtained by keeping the contaminated material in a humid container, where the necrosis affected areas began to show the typical pycnids (with white-yellowish to orange cilia) and pycniospores (α and β type). The expansion of the colonies has been observed daily (by measuring their diameters) as well as the spore-production (macroscopically and microscopically) [17]. The results show that Phomopsis viticola Sacc. developed in acid (4) to basic (11) pH of the growing medium. At the acid pH level (4), the fungus showed limited growth colonies with a low vegetative mass, and from a pH level of 5.5 up to strong basic (11) pH, the fungi developed colonies with an abundant vegetation mass [7]. It has been observed that spores of Phomopsis viticola are produced in a neutral (pH 7.0) to a strong basic pH (10.0–11.0). Spore-production was very good in the GCA medium, good on the malt extract, and absent on the Blakslee and Czapek culture media [17].
In regard to the temperature factor, Podosu et al. [17] observed a very good growth and spore production of the pathogen between 16 °C and 30 °C and placed the optimal growth temperature of Phomopsis viticola between 18 °C and 30 °C. Under low relative humidity conditions (31–38%), the growth of Phomopsis viticola was not substantial (0.1–0.4 cm in diameter), with white, feeble colonies and no spores’ production. At high levels of relative humidity (95%), the colonies became thick, normally developed, and with a good production of spores [17].
The energy sources (carbon source and light influence) of Phomopsis viticola growing in vitro are similar to those described for Eutypa lata. Regarding this matter, Phomopsis viticola showed best development and fructification on melibiosis, zaharosis, cellulose, and lignin growth mediums. Poor development and fructification of Phomopsis viticola was observed on sorbosis and maltosis growth medium. In the absence of the carbon sources, Phomopsis viticola colonies were inhibited [7]. The Phomopsis viticola colonies grown in completely dark conditions have developed the best vegetation mass, and the colonies showed dense, felt-like aspects, but they had weak fructifications. Phomosis viticola had very good fructification in alternative light and dark conditions (8/16 h) [7].
3. Esca in Romania
In Romania, Esca is treated as a vascular disease (the attack of the fungi blocks the xylem, diminishing at first and then completely blocking the circulation of water and nutrients through the vines), caused by several species of fungi, among the most common being Stereum hirsutum (Wild. [1][2][11][21][22]). They severely affect the wood of old and declining vines or those that have undergone periods of drought, extreme temperatures (heat, frost), a poor supply of nutrients, or those which have been planted on heavy soils with poor drainage [2][7][21]. Although Stereum hirsutum is not considered a vascular pathogen, the authors studying Esca in Romania report this fungus as being the main one causing the disease. Stereum hirsutum (Wild.) enters the vines through wood wounds caused by pruning or other accidents. Dead tissue is invaded by the fungus mycelium, which forms the macroconidia (the reproductive organs of the fungus) and sclerotia (the essential elements, the spores along with sclerotia, are released from the spongy mass, which is driven by the spread of the disease). The dry trunks crack, and the wind reaches wood wounds, infecting the tissues on the surface [1][2][11].
In Romania, this GTD manifests sporadically, in very old (20–30 years) vineyards, significant damage being rarely reported (Figure 4).
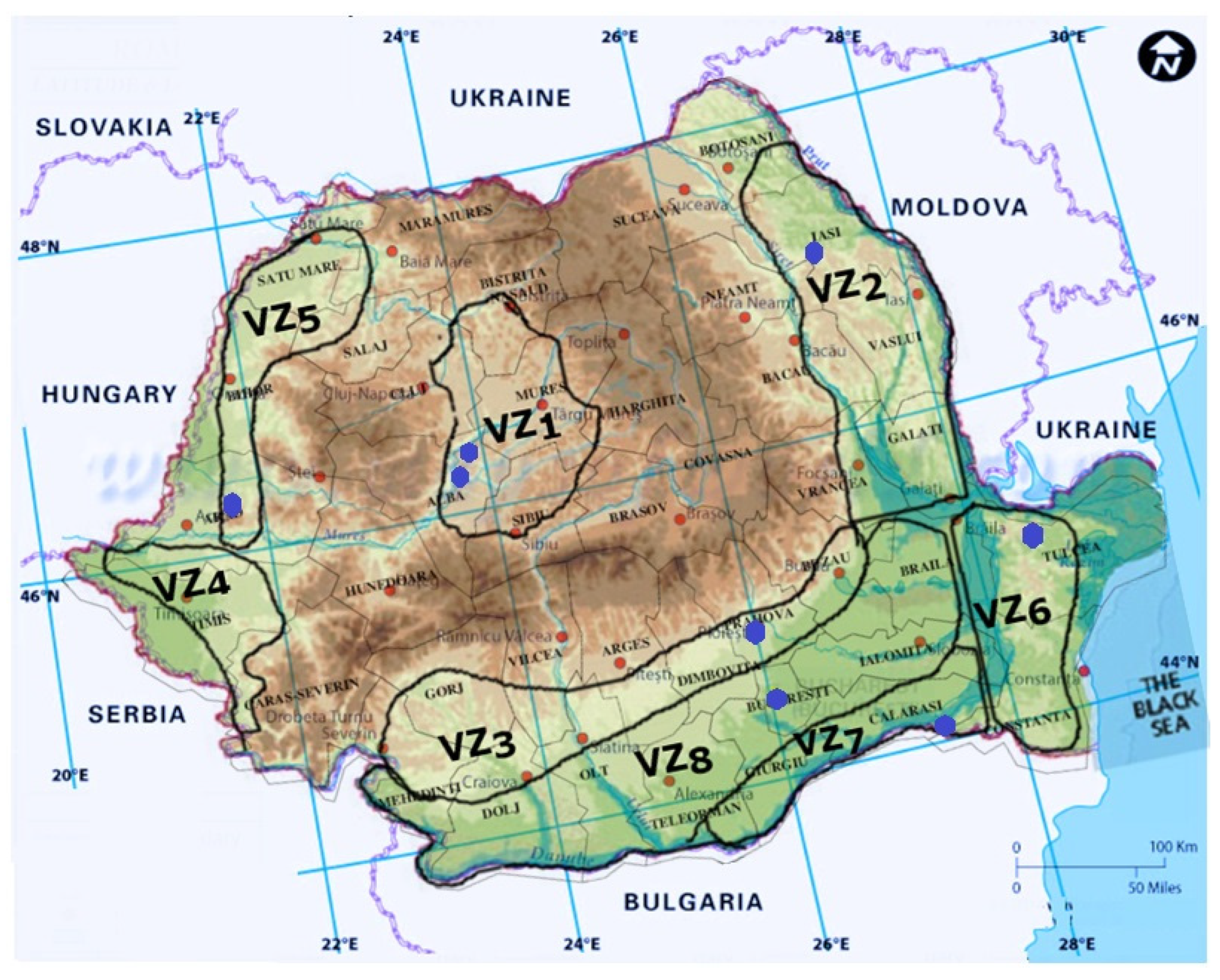
Figure 4. Graphical representation of Esca disease reports in Romania.
3.1. Symptoms
External symptoms have very different aspects because the death of the vine can be slow, over several years, or sudden, by vine withering in a summer [2][11][21][23]. The chronic form, which is manifested on the leaves, is called Grapevine Leaf Stripe Disease (GLSD), and the acute form is called apoplexy [21]. The chronic form is more difficult to diagnose. Grapevine Leaf Stripe Disease (GLSD) begins to manifest in the second half of June, appearing at first on the basal leaves of the vines, in the form of isolated spots, small, chlorotic (in white varieties), or reddish (in red varieties) and irregularly arranged between the main veins. The spots turn necrotic and show a yellow halo between the healthy and affected area. Necrosis also occurs on the edge of the leaves, the attack being more pronounced on the leaves at the base of the shoots. The vines get poorly fed by sap; from year to year, portions of the vine do not start the vegetation period in spring, and finally, the vine dies [1][2][11][21].
Apoplexy is the most severe form of the disease, usually occurring in the hot and dry summer months (July, August) after heavy rains. It manifests as a sudden wilting of the leaves and drying of the entire vines in a very short period of only a few days. The leaves begin to lose their turgidity and dry out from the edge to the base, falling prematurely, while the vine remains bare. The fruit clusters wither and turn brown and remain attached to the canes for a while. The disease is manifested in solitary vines, the attack in outbreaks being very rarely observed [1][2][21].
The symptoms appear every year, but they are more obvious in the dry and hot years [1][2][21]. It is often observed that the vegetation starts from the rootstock or from the basal eyes of the graft [2]. The first symptoms can be observed around the flowering phenophase [1]. In summer, during periods of drought and wind, on the basis of increased evapotranspiration, the shoots and leaves begin to wither, having a reddish-brown appearance [21]. Before entering the varaison phenophase, on grape berries can appear some brown or brunviolaceeous spots. Similar spots also appear on rachis and pedicels. Berries lose their turgidity and begin to raisin. Sometimes their mummification can occur, as well as a longitudinal cracking of infected grains, which are then attacked by insects and rot [21].
The cross section of an Esca infected vine wood has a white-yellowish spongy appearance, surrounded by deep necrotic lesions, which secrete a gummy exudate on cutting. The mycelium of the fungus (Stereum hirsutum) breaks down the wood leading it to rot. The attack progresses year after year, forming annual attack zones, and when it has encompassed the entire circumference of the trunk, apoplectic drying of the vine occurs [1][2][21].
3.2. Fungi Involved
The favoring factors of the appearance and development of Esca infections are: drought, heat, frost, relative humidity of air (more than 25%), optimal temperatures of 20–30 °C, the type of vine training system (Guyot is the most favorable), as well as grape cultivar (very sensitive are Muscat Ottonel, Sauvignon, Cabernet Sauvignon) and vineyards age [2][11][23]. As mentioned, pedoclimatic conditions play an important role in disease evolution [2][7]. Comșa et al. [11] observed that due to heavy rains and extreme temperatures during summer, a more extensive attack of the Esca disease took place in 2010. Oprea and Podosu [7] identified Stereum hirsutum in the Blaj (VZ1), Prahova (VZ3), and Ostrov (VZ7) vineyards, in a proportion of 8%, in a soil showing a high concentration of calcium carbonicum and ferric chloride and strong erosions. The disease of the vine was identified by the specific leaves color and the pathogen by the carpophores’ appearance on the leaves [7]. Moreover, Oprea and Dumitru [2] state that a higher number of dried vines were identified in vineyards with compacted soils. Mature vineyards are more susceptible to Esca, probably due to the higher content of tannins [2]. Matei et al. [23] observed that the type of vine training system can be a favorable factor, and the mode of pruning can favor disease spread. In their study, Esca decline was found in only 2.4% of vines trained by Cazenave cordon, in 3.7% of vines trained by demi-high Guyot as opposed to 13.58% in vines trained by spur-pruned cordon.
Oprea and Dumitru [2] found Esca symptoms in Iași (VZ2) vineyards (Comarna and Uricani), and they associate Esca GTD to Stereum hirsutum (Wild.). Moreover, they observed Muscadet and Cabernet Sauvignon cultivars as strongly attacked by Esca. Matei et al. [23] studied fungal pathogens associated with Esca in a 15 years plantation from Bucharest (VZ8) of Fetească Regală cultivar grafted on Kobber 5 BB and isolated nine fungal species from the wood of the grapevines with Esca symptoms. The isolates were identified according to their morphological characteristics on potato dextrose agar. The fungi isolated from the wood were included in the genera: Phaeoacremonium, Phaeomoniella, Phomopsis, Fomitiporia, Fusarium, Alternaria, Cladosporium, Aspergillus, and Botryosphaeria. Among those, Phaeoacremonium aleophilum and Phaeomoniella chlamydospora occurred at the highest frequencies. Their study suggests that the infection involving the Phaeoacremonium and Phaeomoniella species predisposes the vines to wood rots caused by basidiomycete fungi such as Fomitiporia punctata.
Comșa et al. [11] also link Stereum hirsutum to Esca infected vines from the Viticultural Centre of Blaj (VZ1), and observed that Fetească regală, Italian Riesling, and Sauvignon Blanc cultivars are more susceptible to the attack of lignicole pathogens, and Muscat Ottonel proved to be a more tolerant cultivar. Another study of Comșa et al. [5] states that Stereum hirsutum caused Esca complex on the cultivars Codreanca in Blaj vineyard (VZ1) and Traminer from Ciumbrud vineyard (VZ1). Diplodia seriata was also identified as a pathogen of the Esca complex on the cultivars Sultanina from Cuvin (VZ5) and Victoria from Blaj (VZ1) vineyards. Phaeoacraemonium aleophilum was also found as a pathogen in the infected grapevines of the cultivars Traminer from Ciumbrud (VZ1), Sultanina from Cuvin (VZ5), and Aligote from Sarica Niculitel (VZ6) vineyards, and it is considered to cause Esca complex dieback.
Ulea [1] claims that both Stereum hirsutum (Wild.) and Phellinus igniarius (L. ex Fr.) Quel. are pathogenic fungi isolated from trunks showing typical symptoms of apoplexy, and even if both were isolated in the same samples, one or the other may be more widespread depending on the region.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens11091006
References
- Ulea, E. Declinul Plantatiilor Viticole. ; Ion Ionescu de la Brad: Iași, Romania, 2003; ISBN 973-7921-05-4.
- Oprea, M.; Dumitru, C. Cercetări Privind Ciupercile Patogene Implicate În Declinul Viţei de Vie. . An. ICPP Bucureşti 1989, 22, 11–24.
- Rafailă, C. Excorioza—O Boală Păgubitoare a Viţei de Vie. . Revista de Horticultura si Viticultura 1970, 19, 78–80.
- Comșa, M.; Tomoiagă, L.; Popescu, D.; Cristea, C. Researches Regarding the Eutypa lata Lignicole Fungus Manifestation in Vineyards from Blaj Wine Centre. Bull. UASVM Hortic. 2014, 71, 2.
- Comșa, M.; Tomoiagă, L.; Botea, V.; Sîrbu, A.; Dobromir, D. Identification by Plate Culture Method of the Fungal Pathogens Causing the Grapevine Trunk Diseases in Romanian Vineyards. Rom. J. Hortic. 2021, 2, 137–142.
- Tomoiaga, L.; Chedea, V.S. Grapevine Trunk Diseases Management in Vineyards from Central Transylvania. Bull. USMV Cluj-Napoca. Hortic. 2020, 77, 117–121.
- Oprea, M.; Podosu, A. Grape Dieback in Romania Induced by Pathogenic Lignicoulus Fungi. Fruit Grow. Technol. 2008, 52, 128–133.
- Iacob, V. Bolile Plantelor Cultivate—Prevenire Și Combatere. ; Editura “Ion Ionescu de la Brad”: Iaşi, Romania, 2006; ISBN 632.
- Mirică, I.; Mirică, A. Protecția Viței-de-Vie Împotriva Bolilor Și Dăunătorilor ; Editura CERES: București, Romania, 1986.
- Mirică, I.; Mărmureanu, M.; Oprea, M.; Dumitru, C.; Mirică, A.; Filip, I.; Julei, S. Researches on Bioecology, Transmission and Control of Eutypa lata Fungi. Prod. Veg. Hortic. 1989, 2, 35–39.
- Comșa, M.L.; Cudur, F.; Cudur, C.; Cristea, C. Research on Some Pathogenic Fungi Involved in the Biological Decline of the Grapevine at the Blaj Viticultural Centre. In Seria Horticultură; Universitatea de Științe Agricole și Medicină Veterinară “Ion Ionescu de la Brad”: Iași, Romania, 2012; pp. 503–508.
- Mărmureanu, M.; Dumitru, C.; Oprea, M. Bioecologia, Transmiterea Şi Combaterea Ciupercii Phomopsis viticola Sacc. . Horticultura 1990, 39, 26–30.
- Tică, C.; Şesan, T.; Oprea, M. Cercetări Privind Biologia Şi Combaterea Excoriozei Viţei de Vie (Phomopsis viticola Sacc.), În Codiţiile Podgoriilor Din Vrancea. ; Institutul de Cercetare Dezvoltare Pentru Viticultura si Vinificatie: Călugărească, Romania, 1994; Volume 14.
- Tică, C.; Şesan, T.; Oprea, M. Excorioza (Phomopsis viticola), Boală de Importanţă Majoră a Viţei de Vie. . Probleme Protecţia Plantelor 1994, 22, 21–51.
- Ulea, V. Contribuţii La Studiul Ciupercilor Parazite Şi Saprofite Care Atacă Scoarţa Şi Lemnul Butucilor de Viţă de Vie Cu Privire Specială Asupra Excoriozei, Produsă de Phomopsis viticola Sacc. ; USAMV: Iaşi, Romania, 1997.
- Savu, S.; Tomoiaga, L.L.; Chedea, V.S. Ecological Microclimate Influence on Grapevine Phomopsis viticola Attack Frequency in Aiud-Ciumbrud Vineyards. Bull. USAMV Cluj-Napoca. Hortic. 2020, 77, 1843–5394.
- Podosu, A.; Mihu, G.; Stoian, I. Research Concerning the Biology of the Grapevine Excoriosis (Phomopsis viticola SACC.) under the Conditions of the Vineyards in Vrancea. Lucr. Științifice USAMV Iași Ser. Hortic. 2008, 51, 1197–1204.
- Botea, V.; Tomoiagă, L.L.; Vasiu, I.; Sîrbu, A.; Răcoare, H.S.; Chedea, V.S. Phomopsis viticola Management in Târnave Vineyards. Rom. J. Hortic. 2022; in press.
- Bulit, J.; Bugaret, Y.; Verdu, D. On the Possibilities on Conservation in Winter of Botrytis Cinerea Pers. and Phomopsis viticola Sacc. in the Buds of the Grapevine. Revue Zool. Agricole et Pathol. Vegetale 1973, 1, 1–12.
- Bugaret, Y. New Facts on Phomopsis Cane Blight and Leaf Spot Epidemiology and Their Consequences for the Control-French. Phytoma 1986, 375, 36–41.
- ANF-Autoritatea Naționlă Fitosanitară (Romanian national phytosanitary authority). Ghid Pentru Recunoașterea Și Combaterea Bolilor Și Dăunătorilor La Vița de Vie ; Autoritatea Naţională Fitosanitară: Voluntari, Romania, 2016.
- Tomoiagă, L.; Oroian, I. Identifying and Setting the Parameters of the Risk Factors Involved in the Process of Biological Decline of the Vines in Romania. J. Environ. Prot. Ecol. 2012, 13, 1350–1356.
- Matei, P.; Iacomi, B.; Dragan, G. Fungi Associated with Esca Decline and Their in Vitro Control by Chitosan. Sci. Pap. UASVM Buchar. 2010, 53, 448–453.
This entry is offline, you can click here to edit this entry!
