Cyclodextrins (CDs) are biocompatible and accepted in biological applications; therefore, there is a growing interest in them both scientifically and industrially. CDs are obtained through the enzymatic degradation of potatoes, corn and rice starch, which gives a mixture of linear, branched, or cyclic dextrins. CDs have a very important role in textile processing and innovation; their use provides immediate opportunities for the development of environmentally friendly products and eco-textiles, in addition to having great potential in various applications. Cyclodextrins can be applied in the areas of spinning, pretreatment, dyeing, finishing, and dye removal, with dyeing, finishing, and water treatment being the most applicable in the textile area registered so far.
- cyclodextrin
- dyeing
- textile finishing
1. Dyeing Process
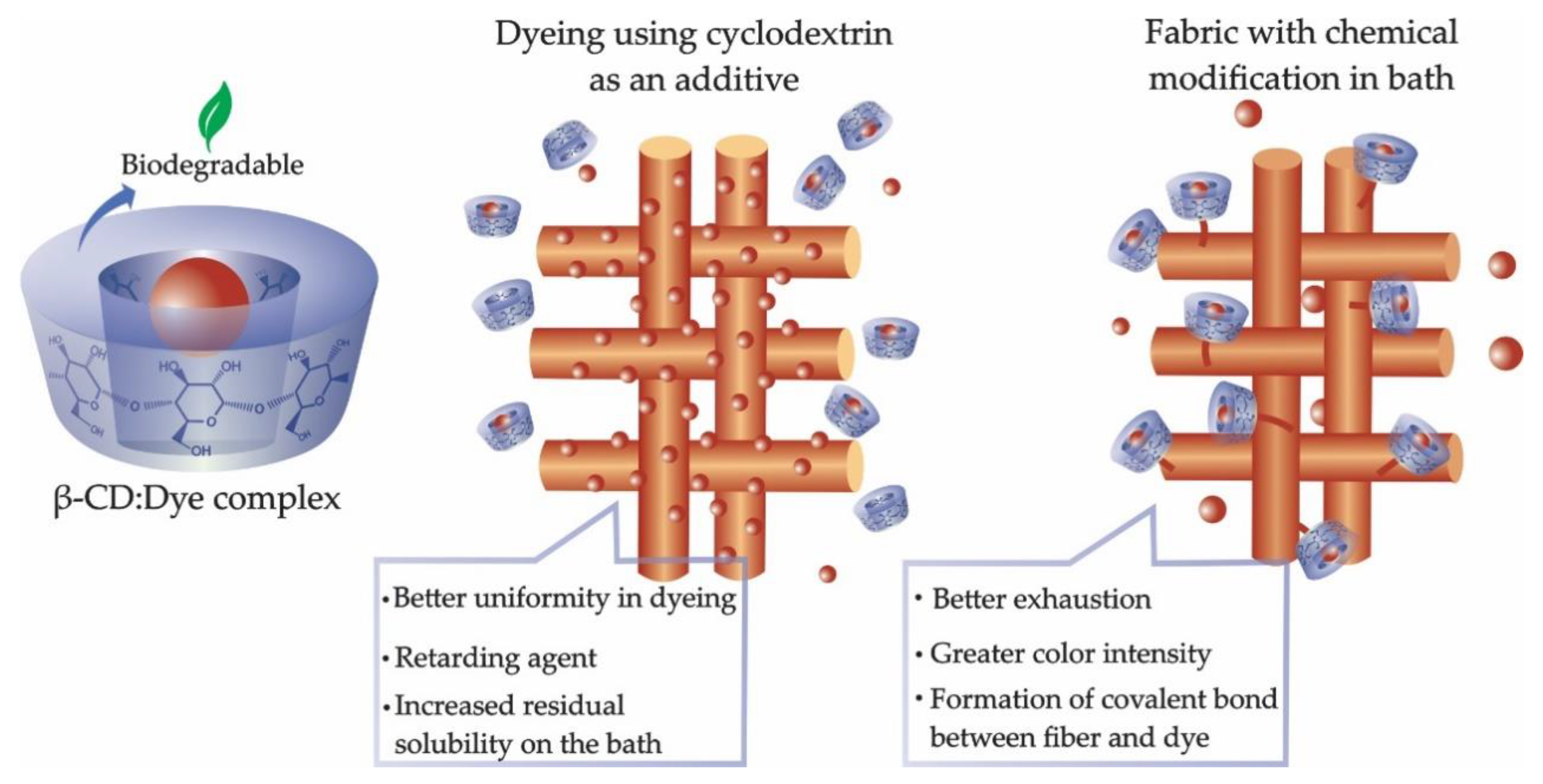
1.1. Cyclodextrin as an Auxiliary Agent in Dyeing
1.2. Dyeing Chemical Modification
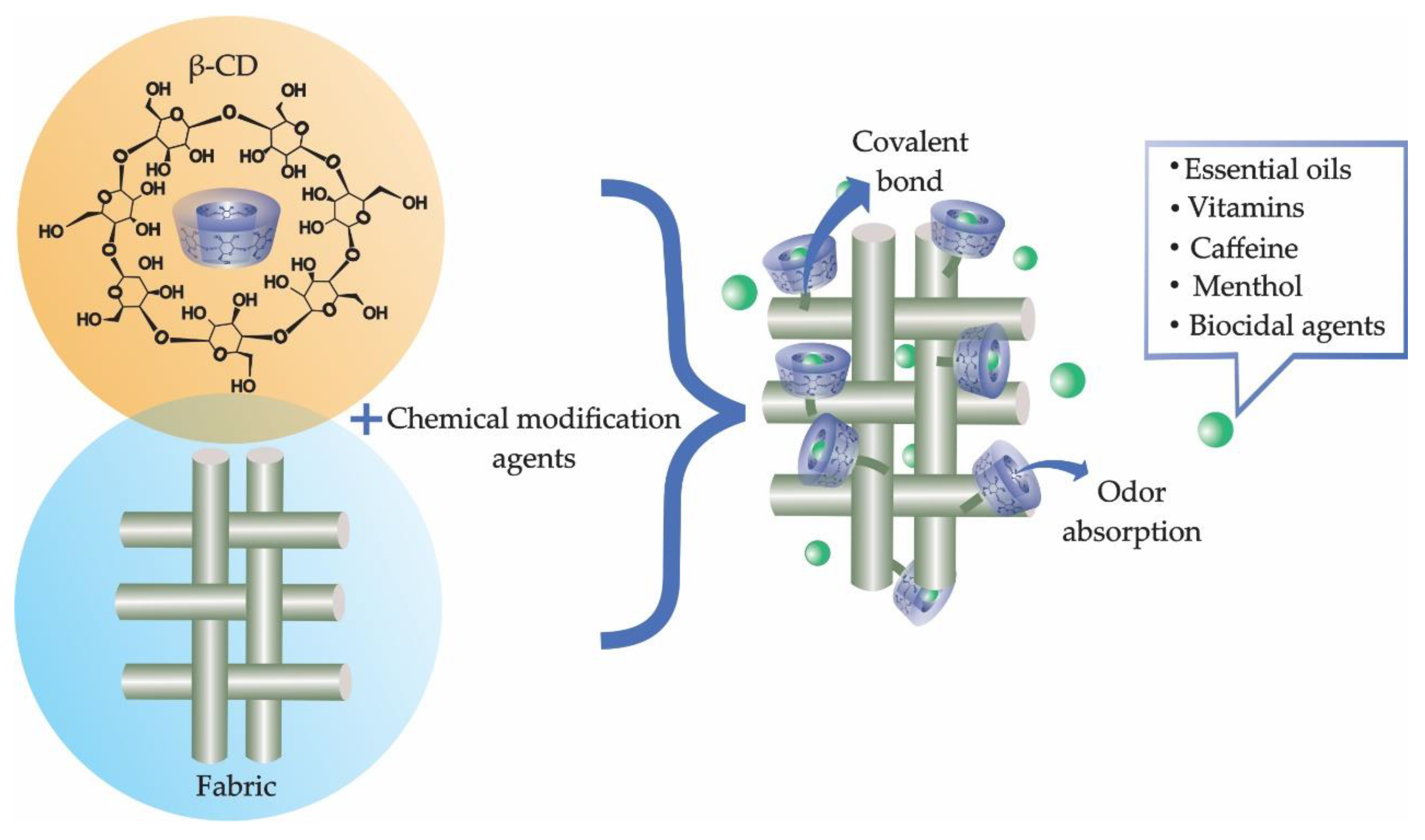
2. Textile Finishing
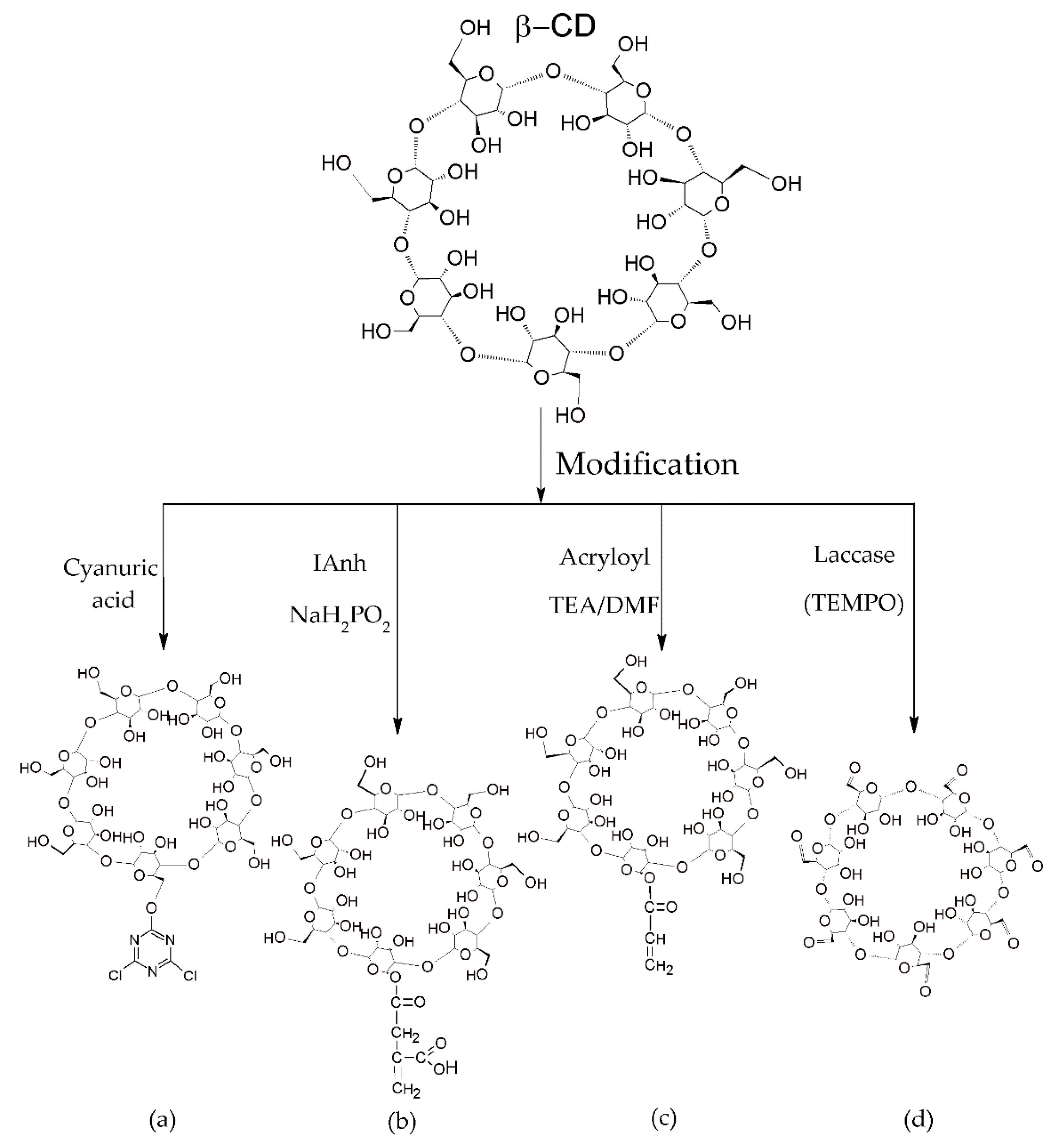
2.1. Preparation of Cyclodextrins
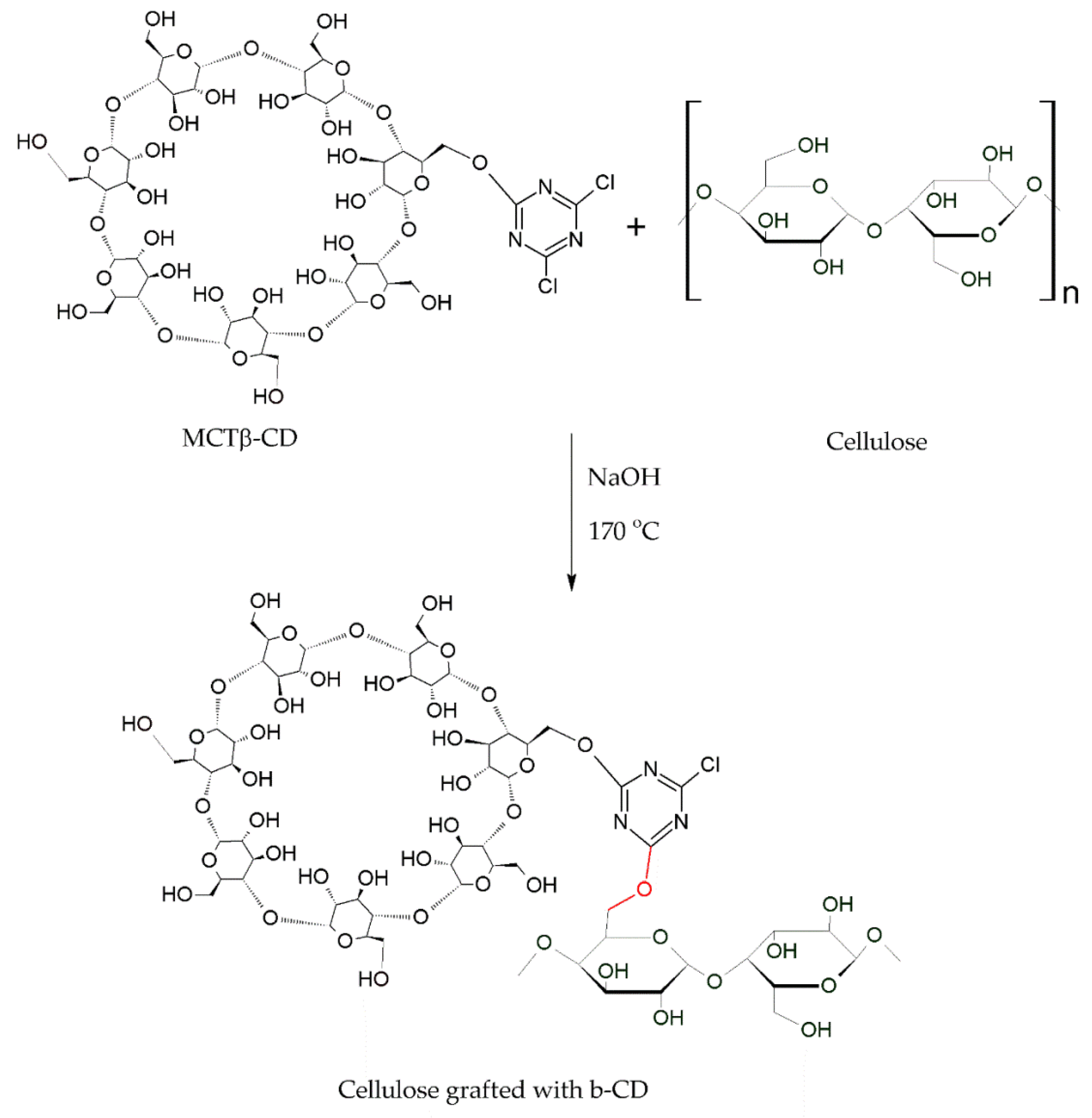
2.2. Grafting of Cyclodextrins onto Textile Substrates
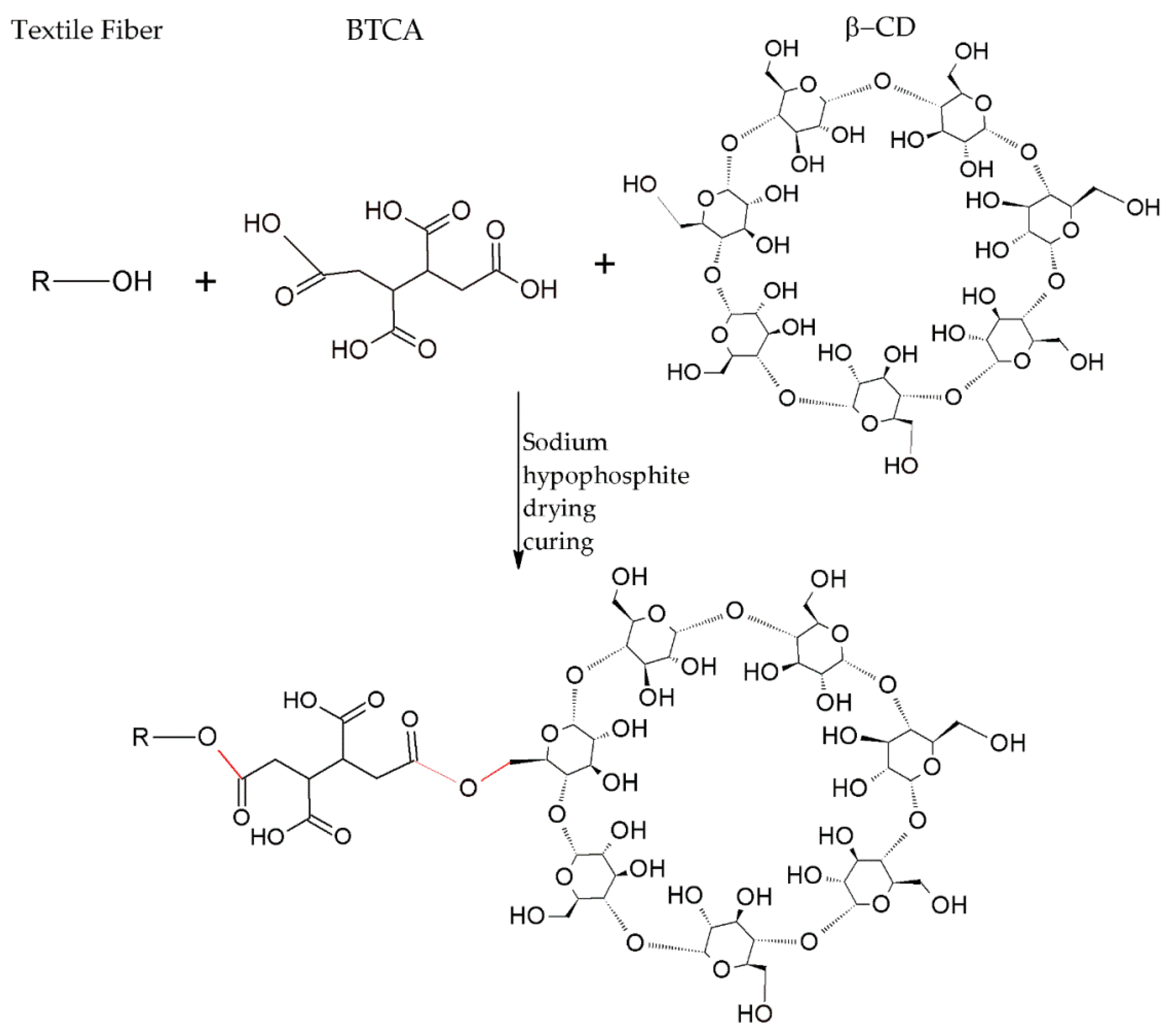
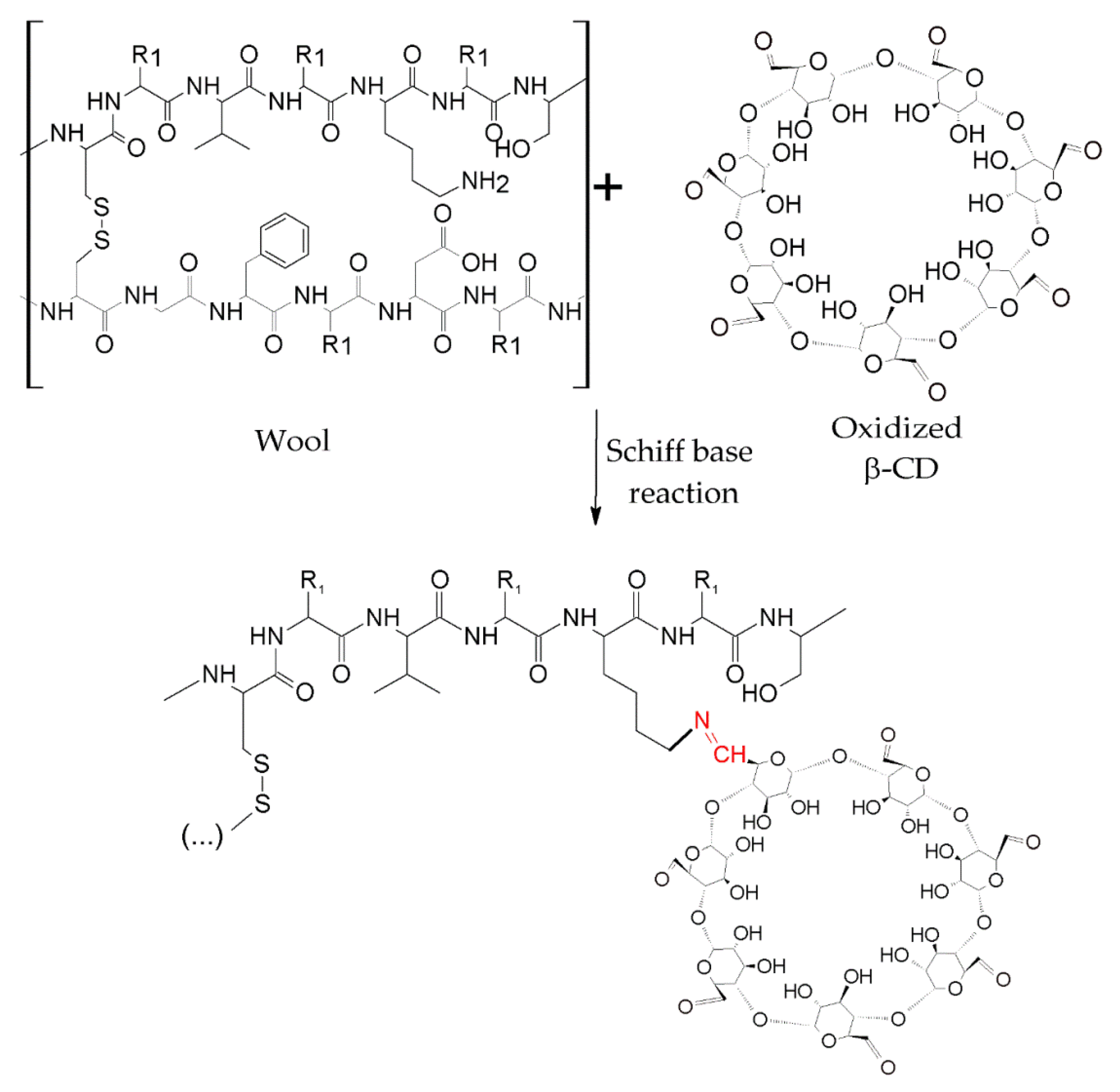
3. New Trends in Textile Finishes Using Cyclodextrins
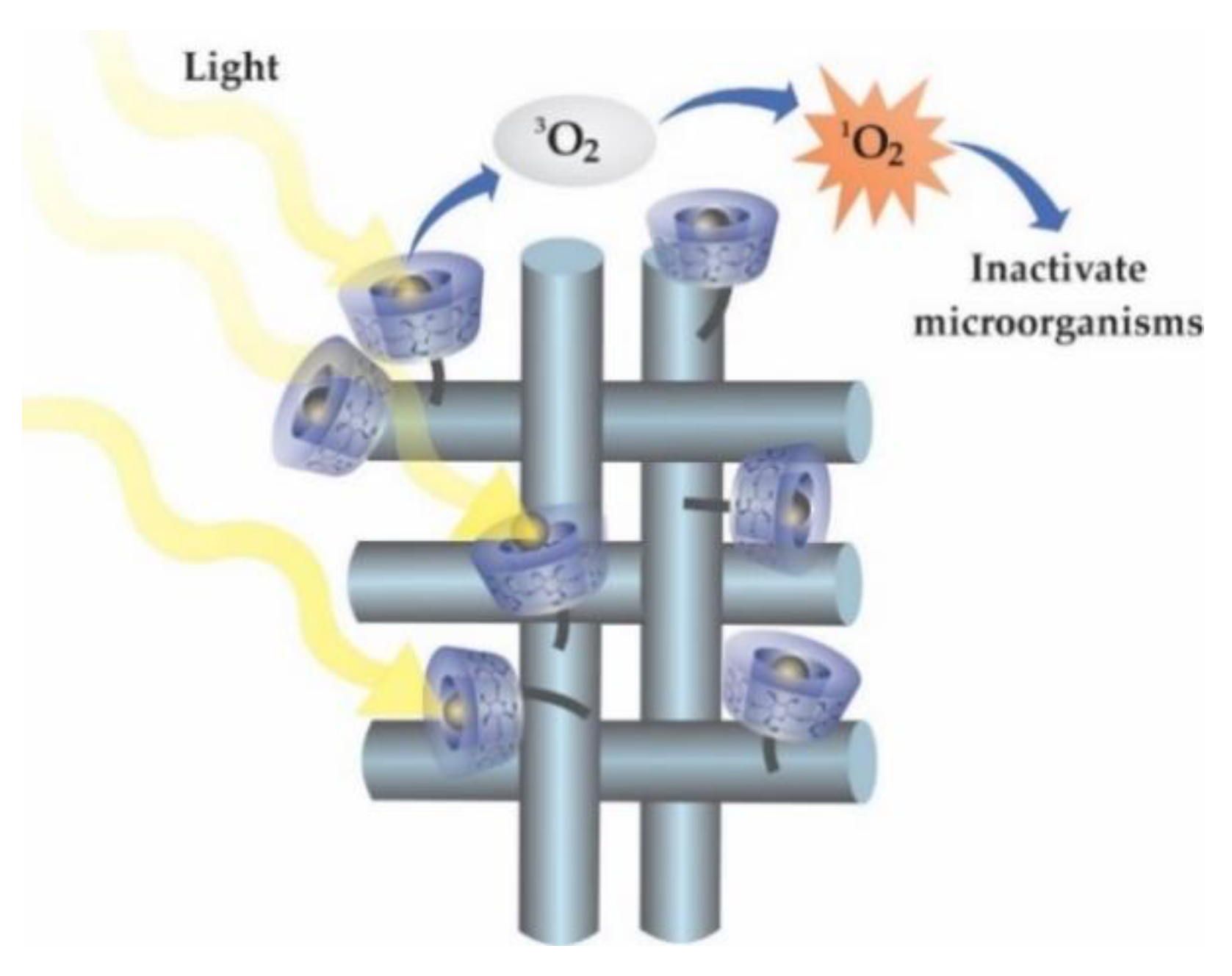
4. Cyclodextrins in Textile Effluent Treatment
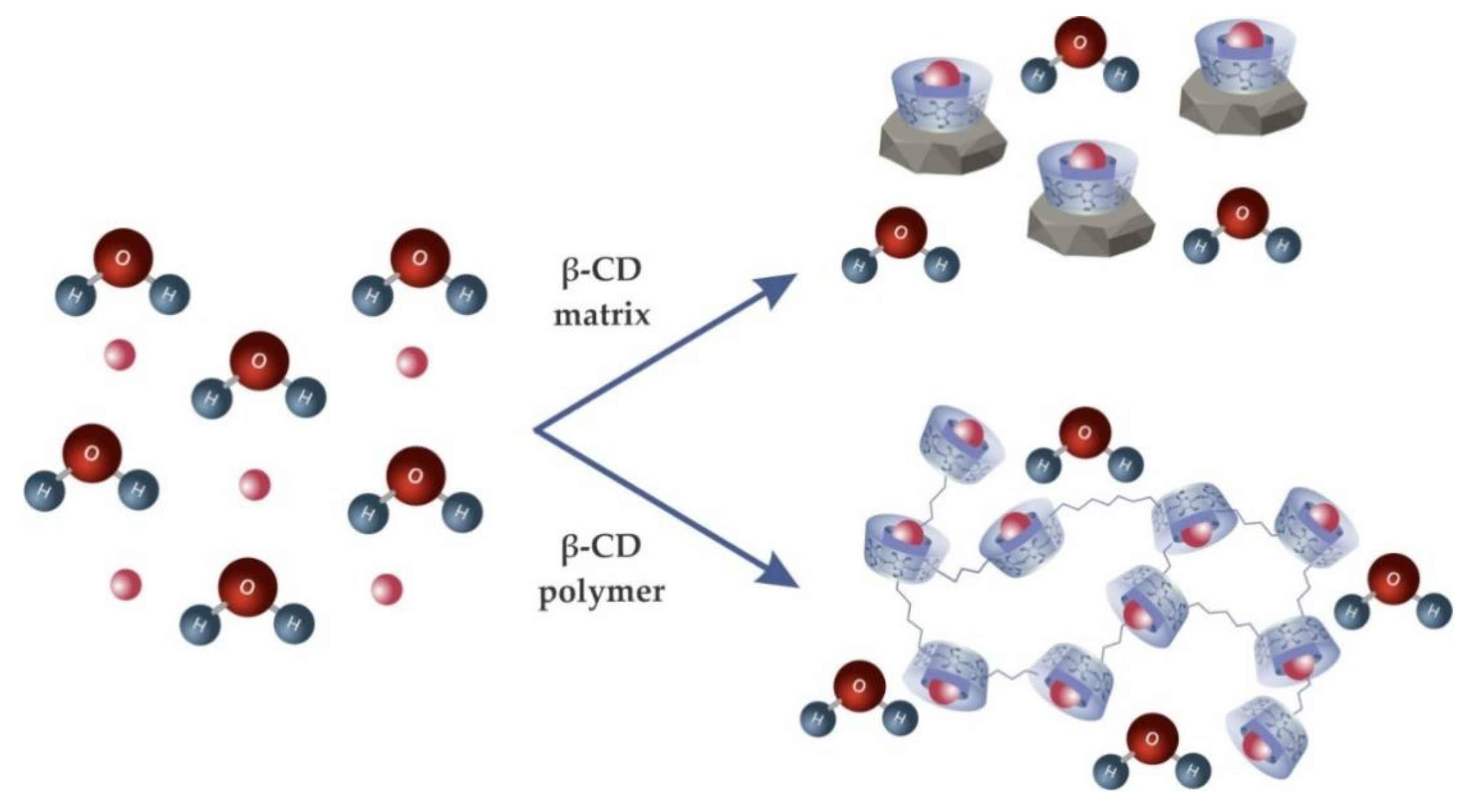
4.1. Cyclodextrin Matrix
4.2. Cyclodextrin Polymers
This entry is adapted from the peer-reviewed paper 10.3390/molecules25163624
References
- Bezerra, F.M.; Moraes, F.F.D.; Santos, W.L.F.; Santos, J.C. Emprego de β-ciclodextrina como auxiliar no tingimento de fibras têxteis. Química Têxt. 2013, 27, 12–21.
- Cireli, A.; Yurdakul, B. Application of cyclodextrin to the textile dyeing and washing processes. J. Appl. Polym. Sci. 2006, 100, 208–218.
- Park, J.S.; Kim, I.-S. Use of β-cyclodextrin in an antimigration coating for polyester fabric. Color. Technol. 2013, 129, 347–351.
- Kacem, I.; Laurent, T.; Blanchemain, N.; Neut, C.; Chai, F.; Haulon, S.; Hildebrand, H.F.; Martel, B. Dyeing and antibacterial activation with methylene blue of a cyclodextrin modified polyester vascular graft. J. Biomed. Mater. Res. Part A 2014, 102, 2942–2951.
- Andreaus, J.; Dalmolin, M.C.; Junior, I.B.D.O.; Barcellos, I.O. Application of cyclodextrins in textile processes. Química Nova 2010, 33, 929–937.
- Buschmann, H.-J.; Denter, U.; Knittel, D.; Schollmeyer, E. The Use of Cyclodextrins in Textile Processes—An Overview. J. Text. Inst. 1998, 89, 554–561.
- Bhaskara-Amrit, U.R.; Agrawal, P.B.; Warmoeskerken, M.M.C.G. Applications of β-cyclodextrins in textiles. Autex Res. J. 2011, 11, 94–101.
- Dutra, F.V.; Santos, K.R.M.D.; Bezerra, F.M. Complexação de corantes dispersos utilizando ß-ciclodextrina em tricromia para poliéster. Química Têxt. 2019, 43, 40–49.
- Raslan, W.M.; El-Aref, A.T.; Bendak, A. Modification of cellulose acetate fabric with cyclodextrin to improve its dyeability. J. Appl. Polym. Sci. 2009, 112, 3192–3198.
- Lu, M.; Liu, Y.P. Dyeing Kinetics of Vinylon Modified with β-Cyclodextrin. Fibres Text. East. Eur. 2011, 5, 88.
- Burkinshaw, S.M.; Liu, K.; Salihu, G. The wash-off of dyeings using interstitial water Part 5: Residual dyebath and wash-off liquor generated during the application of disperse dyes and reactive dyes to polyester/cotton fabric. Dye. Pigment. 2019, 171, 106367.
- Carpignano, R.; Parlati, S.; Piccinini, P.; Savarino, P.; Giorgi, M.R.D.; Fochi, R. Use of β-cyclodextrin in the dyeing of polyester with low environmental impact. Color. Technol. 2010, 126, 201–208.
- Dardeer, H.M.; El-sisi, A.A.; Emam, A.A.; Hilal, N.M. Synthesis, Application of a Novel Azo Dye and Its Inclusion Complex with Beta-cyclodextrin onto Polyester Fabric. Int. J. Text. Sci. 2017, 6, 79–87.
- Xie, K.; Liu, H.; Wang, X. Surface modification of cellulose with triazine derivative to improve printability with reactive dyes. Carbohydr. Polym. 2009, 78, 538–542.
- Burkinshaw, S.M.; Mignanelli, M.; Froehling, P.E.; Bide, M.J. The use of dendrimers to modify the dyeing behaviour of reactive dyes on cotton. Dye. Pigment. 2000, 47, 259–267.
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres part 2: Analysis of conventional models that describe the manner by which inorganic electrolytes promote direct dye uptake on cellulosic fibres. Dye. Pigment. 2019, 161, 531–545.
- Burkinshaw, S.M.; Salihu, G. The role of auxiliaries in the immersion dyeing of textile fibres: Part 4 theoretical model to describe the role of liquor ratio in dyeing cellulosic fibres with direct dyes in the absence and presence of inorganic electrolyte. Dye. Pigment. 2019, 161, 565–580.
- Parlati, S.; Gobetto, R.; Barolo, C.; Arrais, A.; Buscaino, R.; Medana, C.; Savarino, P. Preparation and application of a β-cyclodextrin-disperse/reactive dye complex. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 463–470.
- Vončina, B.; Vivod, V.; Jaušovec, D. β-Cyclodextrin as retarding reagent in polyacrylonitrile dyeing. Dye. Pigment. 2007, 74, 642–646.
- Shibusawa, T.; Okamoto, J.; Abe, K.; Sakata, K.; Ito, Y. Inclusion of azo disperse dyes by cyclodextrins at dyeing temperature. Dye. Pigment. 1998, 36, 79–91.
- Ghoul, Y.E.; Martel, B.; Achari, A.E.; Campagne, C.; Razafimahefa, L.; Vroman, I. Improved dyeability of polypropylene fabrics finished with β-cyclodextrin–citric acid polymer. Polym. J. 2010, 42, 804–811.
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley-VCH: Weinheim, Germany, 2003; ISBN 978-3-527-30426-4.
- Wang, H.; Lewis, D.M. Chemical modification of cotton to improve fibre dyeability. Color. Technol. 2002, 118, 159–168.
- Udrescu, C.; Ferrero, F.; Periolatto, M. Ultrasound-assisted dyeing of cellulose acetate. Ultrason. Sonochem. 2014, 21, 1477–1481.
- Zhang, W.; Ji, X.; Wang, C.; Yin, Y. One-bath one-step low-temperature dyeing of polyester/cotton blended fabric with cationic dyes via β-cyclodextrin modification. Text. Res. J. 2019, 89, 1699–1711.
- Ibrahim, N.A.; El-Zairy, E.M.R. Union disperse printing and UV-protecting of wool/polyester blend using a reactive β-cyclodextrin. Carbohydr. Polym. 2009, 76, 244–249.
- Chen, L.; Wang, C.; Tian, A.; Wu, M. An attempt of improving polyester inkjet printing performance by surface modification using β-cyclodextrin. Surf. Interface Anal. 2012, 44, 1324–1330.
- Martel, B.; Ruffin, D.; Weltrowski, M.; Lekchiri, Y.; Morcellet, M. Water-soluble polymers and gels from the polycondensation between cyclodextrins and poly(carboxylic acid)s: A study of the preparation parameters. J. Appl. Polym. Sci. 2005, 97, 433–442.
- Rehan, M.; Mahmoud, S.A.; Mashaly, H.M.; Youssef, B.M. β-Cyclodextrin assisted simultaneous preparation and dyeing acid dyes onto cotton fabric. React. Funct. Polym. 2020, 151, 104573.
- Lis, M.J.; Carmona, O.G.; Carmona, C.G.; Bezerra, F.M. Inclusion Complexes of Citronella Oil with β-Cyclodextrin for Controlled Release in Biofunctional Textiles. Polymers 2018, 10, 1324.
- Scacchetti, F.A.P.; Pinto, E.; Soares, G.M.B. Functionalization and characterization of cotton with phase change materials and thyme oil encapsulated in beta-cyclodextrins. Prog. Org. Coatings 2017, 107, 64–74.
- Bezerra, F.M.; Carmona, O.G.; Carmona, C.G.; Plath, A.S.; Lis, M. Biofunctional wool using β-cyclodextrins as vehiculizer of citronella oil. Process. Biochem. 2019, 77, 151–158.
- Arias, M.J.L.; Coderch, L.; Martí, M.; Alonso, C.; Carmona, O.G.; Carmona, C.G.; Maesta, F. Vehiculation of Active Principles as a Way to Create Smart and Biofunctional Textiles. Materials 2018, 11, 2152.
- Ciobanu, A.; Mallard, I.; Landy, D.; Brabie, G.; Nistor, D.; Fourmentin, S. Retention of aroma compounds from Mentha piperita essential oil by cyclodextrins and crosslinked cyclodextrin polymers. Food Chem. 2013, 138, 291–297.
- Peila, R.; Migliavacca, G.; Aimone, F.; Ferri, A.; Sicardi, S. A comparison of analytical methods for the quantification of a reactive β-cyclodextrin fixed onto cotton yarns. Cellulose 2012, 19, 1097–1105.
- Mihailiasa, M.; Caldera, F.; Li, J.; Peila, R.; Ferri, A.; Trotta, F. Preparation of functionalized cotton fabrics by means of melatonin loaded β-cyclodextrin nanosponges. Carbohydr. Polym. 2016, 142, 24–30.
- Nardello-Rataj, V.; Leclercq, L. Encapsulation of biocides by cyclodextrins: Toward synergistic effects against pathogens. Beilstein J. Org. Chem. 2014, 10, 2603–2622.
- Hedayati, N.; Montazer, M.; Mahmoudirad, M.; Toliyat, T. Ketoconazole and Ketoconazole/β-cyclodextrin performance on cotton wound dressing as fungal skin treatment. Carbohydr. Polym. 2020, 240, 116267.
- El-Ghoul, Y. Biological and microbiological performance of new polymer-based chitosan and synthesized amino-cyclodextrin finished polypropylene abdominal wall prosthesis biomaterial. Text. Res. J. 2020, 0040517520926624.
- McQueen, R.H.; Vaezafshar, S. Odor in textiles: A review of evaluation methods, fabric characteristics, and odor control technologies. Text. Res. J. 2019, 90, 1–17.
- Kadam, V.; Kyratzis, I.L.; Truong, Y.B.; Wang, L.; Padhye, R. Air filter media functionalized with β-Cyclodextrin for efficient adsorption of volatile organic compounds. J. Appl. Polym. Sci. 2020, 137, 49228.
- Leclercq, L. 17 - Smart medical textiles based on cyclodextrins for curative or preventive patient care. In Active Coatings for Smart Textiles; Hu, J., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2016; pp. 391–427. ISBN 978-0-08-100263-6.
- Azizi, N.; Ben Abdelkader, M.; Chevalier, Y.; Majdoub, M. New β-Cyclodextrin-Based Microcapsules for Textiles Uses. Fibers Polym. 2019, 20, 683–689.
- Wang, C.X.; Chen, S.L. Aromachology and its application in the textile field. Fibres Text. East. Eur. 2005, 13, 41–44.
- Shabbir, M.; Ahmed, S.; Sheikh, J.N. Frontiers of Textile Materials: Polymers, Nanomaterials, Enzymes, and Advanced Modification Techniques; Scrivener Publishing: Beverly, MA, USA, 2020; ISBN 978-1-119-62036-5.
- Agrawal, P.B.; Warmoeskerken, M.M.C.G. Permanent fixation of β-cyclodextrin on cotton surface—An assessment between innovative and established approaches. J. Appl. Polym. Sci. 2012, 124, 4090–4097.
- Nazi, M.; Malek, R.M.A.; Kotek, R. Modification of β-cyclodextrin with itaconic acid and application of the new derivative to cotton fabrics. Carbohydr. Polym. 2012, 88, 950–958.
- Zhang, F.; Islam, M.S.; Berry, R.M.; Tam, K.C. β-Cyclodextrin-Functionalized Cellulose Nanocrystals and Their Interactions with Surfactants. ACS Omega 2019, 4, 2102–2110.
- Reuscher, H.; Hirsenkorn, R. BETA W7 MCT—New ways in surface modification. J. Incl. Phenom. Macrocycl. Chem. 1996, 25, 191–196.
- Shown, I.; Murthy, C.N. Grafting of cotton fiber by water-soluble cyclodextrin-based polymer. J. Appl. Polym. Sci. 2009, 111, 2056–2061.
- Radu, C.-D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles—Review. J. Control. Release 2016, 224, 146–157.
- Nichifor, M.; Constantin, M.; Mocanu, G.; Fundueanu, G.; Branisteanu, D.; Costuleanu, M.; Radu, C.D. New multifunctional textile biomaterials for the treatment of leg venous insufficiency. J. Mater. Sci. Mater. Med. 2009, 20, 975–982.
- Yu, Y.; Wang, Q.; Yuan, J.; Fan, X.; Wang, P. A novel approach for grafting of β-cyclodextrin onto wool via laccase/TEMPO oxidation. Carbohydr. Polym. 2016, 153, 463–470.
- Haji, A.; Mehrizi, M.K.; Akbarpour, R. Optimization of β-cyclodextrin grafting on wool fibers improved by plasma treatment and assessment of antibacterial activity of berberine finished fabric. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 121–133.
- Montazer, M.; Jolaei, M.M. β-Cyclodextrin stabilized on three-dimensional polyester fabric with different crosslinking agents. J. Appl. Polym. Sci. 2010, 116, 210–217.
- Abdel-Halim, E.S.; Abdel-Mohdy, F.A.; Al-Deyab, S.S.; El-Newehy, M.H. Chitosan and monochlorotriazinyl-β-cyclodextrin finishes improve antistatic properties of cotton/polyester blend and polyester fabrics. Carbohydr. Polym. 2010, 82, 202–208.
- Shi, H.; Wang, Y.; Hua, R. Acid-catalyzed carboxylic acid esterification and ester hydrolysis mechanism: Acylium ion as a sharing active intermediate via a spontaneous trimolecular reaction based on density functional theory calculation and supported by electrospray ionization-mass spectrometry. Phys. Chem. Chem. Phys. 2015, 17, 30279–30291.
- Radu, C.-D.; Salariu, M.; Avadanei, M.; Ghiciuc, C.; Foia, L.; Lupusoru, E.C.; Ferri, A.; Ulea, E.; Lipsa, F. Cotton-made cellulose support for anti-allergic pajamas. Carbohydr. Polym. 2013, 95, 479–486.
- Khanna, S.; Chakraborty, J.N. Optimization of monochlorotriazine β-cyclodextrin grafting on cotton and assessment of release behavior of essential oils from functionalized fabric. Fash. Text. 2017, 4, 6.
- Ibrahim, N.A.; Abdalla, W.A.; El-Zairy, E.M.R.; Khalil, H.M. Utilization of monochloro-triazine β-cyclodextrin for enhancing printability and functionality of wool. Carbohydr. Polym. 2013, 92, 1520–1529.
- Popescu, V.; Vasluianu, E.; Popescu, G. Quantitative analysis of the multifunctional finishing of cotton fabric with non-formaldehyde agents. Carbohydr. Polym. 2014, 111, 870–882.
- Martel, B.; Weltrowski, M.; Ruffin, D.; Morcellet, M. Polycarboxylic acids as crosslinking agents for grafting cyclodextrins onto cotton and wool fabrics: Study of the process parameters. J. Appl. Polym. Sci. 2002, 83, 1449–1456.
- Liu, S. Bio-Functional Textiles. In Handbook of Medical Textiles; Textiles; Woodhead Publishing: Cambridge, UK, 2011; pp. 336–359. ISBN 978-0-85709-369-1.
- Blanchemain, N.; Karrout, Y.; Tabary, N.; Neut, C.; Bria, M.; Siepmann, J.; Hildebrand, H.F.; Martel, B. Methyl-β-cyclodextrin modified vascular prosthesis: Influence of the modification level on the drug delivery properties in different media. Acta Biomater. 2011, 7, 304–314.
- Castriciano, M.A.; Zagami, R.; Casaletto, M.P.; Martel, B.; Trapani, M.; Romeo, A.; Villari, V.; Sciortino, M.T.; Grasso, L.; Guglielmino, S.; et al. Poly(carboxylic acid)-Cyclodextrin/Anionic Porphyrin Finished Fabrics as Photosensitizer Releasers for Antimicrobial Photodynamic Therapy. Biomacromolecules 2017, 18, 1134–1144.
- Yao, T.; Wang, J.; Xue, Y.; Yu, W.; Gao, Q.; Ferreira, L.; Ren, K.-F.; Ji, J. A photodynamic antibacterial spray-coating based on the host–guest immobilization of the photosensitizer methylene blue. J. Mater. Chem. B 2019, 7, 5089–5095.
- Hu, X.; Hu, Y.; Xu, G.; Li, M.; Zhu, Y.; Jiang, L.; Tu, Y.; Zhu, X.; Xie, X.; Li, A. Green synthesis of a magnetic β-cyclodextrin polymer for rapid removal of organic micro-pollutants and heavy metals from dyeing wastewater. Environ. Res. 2020, 180, 108796.
- Jiang, H.-L.; Xu, M.-Y.; Xie, Z.-W.; Hai, W.; Xie, X.-L.; He, F.-A. Selective adsorption of anionic dyes from aqueous solution by a novel β-cyclodextrin-based polymer. J. Mol. Struct. 2020, 1203, 127373.
- Chen, J.; Liu, M.; Pu, Y.; Wang, C.; Han, J.; Jiang, M.; Liu, K. The preparation of thin-walled multi-cavities β-cyclodextrin polymer and its static and dynamic properties for dyes removal. J. Environ. Manag. 2019, 245, 105–113.
- Chang, Y.; Dou, N.; Liu, M.; Jiang, M.; Men, J.; Cui, Y.; Li, R.; Zhu, Y. Efficient removal of anionic dyes from aqueous solution using CTAB and β-cyclodextrin-induced dye aggregation. J. Mol. Liq. 2020, 309, 113021.
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution. Desalination 2010, 263, 240–248.
- Zare, K.; Gupta, V.K.; Moradi, O.; Makhlouf, A.S.H.; Sillanpää, M.; Nadagouda, M.N.; Sadegh, H.; Shahryari-ghoshekandi, R.; Pal, A.; Wang, Z.; et al. A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: A review. J. Nanostruct. Chem. 2015, 5, 227–236.
- Sansuk, S.; Srijaranai, S.; Srijaranai, S. A New Approach for Removing Anionic Organic Dyes from Wastewater Based on Electrostatically Driven Assembly. Environ. Sci. Technol. 2016, 50, 6477–6484.
- Debnath, S.; Ballav, N.; Maity, A.; Pillay, K. Competitive adsorption of ternary dye mixture using pine cone powder modified with β-cyclodextrin. J. Mol. Liq. 2017, 225, 679–688.
- Abd-Elhamid, A.I.; El-Aassar, M.R.; El Fawal, G.F.; Soliman, H.M.A. Fabrication of polyacrylonitrile/β-cyclodextrin/graphene oxide nanofibers composite as an efficient adsorbent for cationic dye. Environ. NanoTechnol. Monit. Manag. 2019, 11, 100207.
- Batista, L.M.B.; dos Santos, A.J.; da Silva, D.R.; de Melo Alves, A.P.; Garcia-Segura, S.; Martínez-Huitle, C.A. Solar photocatalytic application of NbO2OH as alternative photocatalyst for water treatment. Sci. Total Environ. 2017, 596–597, 79–86.
- Basha, C.A.; Selvakumar, K.V.; Prabhu, H.J.; Sivashanmugam, P.; Lee, C.W. Degradation studies for textile reactive dye by combined electrochemical, microbial and photocatalytic methods. Sep. Purif. Technol. 2011, 79, 303–309.
- Chollom, M.N.; Rathilal, S.; Pillay, V.L.; Alfa, D. The applicability of nanofiltration for the treatment and reuse of textile reactive dye effluent. Water SA 2015, 41, 398–405.
- Yeap, K.L.; Teng, T.T.; Poh, B.T.; Morad, N.; Lee, K.E. Preparation and characterization of coagulation/flocculation behavior of a novel inorganic–organic hybrid polymer for reactive and disperse dyes removal. Chem. Eng. J. 2014, 243, 305–314.
- Gosavi, V.D.; Sharma, S. A General Review on Various Treatment Methods for Textile Wastewater. Available online: /paper/A-General-Review-on-Various-Treatment-Methods-for-Gosavi-Sharma/e8d649881280265b4bf146b25f6e5eb2ddb99afb (accessed on 14 June 2020).
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Thomaidis, N.S.; Xu, J. Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: A critical review. J. Hazard. Mater. 2017, 323, 274–298.
- Lin, Q.; Gao, M.; Chang, J.; Ma, H. Adsorption properties of crosslinking carboxymethyl cellulose grafting dimethyldiallylammonium chloride for cationic and anionic dyes. Carbohydr. Polym. 2016, 151, 283–294.
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dye. Pigment. 2008, 77, 415–426.
- Pellicer, J.A.; Rodríguez-López, M.I.; Fortea, M.I.; Lucas-Abellán, C.; Mercader-Ros, M.T.; López-Miranda, S.; Gómez-López, V.M.; Semeraro, P.; Cosma, P.; Fini, P.; et al. Adsorption Properties of β- and Hydroxypropyl-β-Cyclodextrins Cross-Linked with Epichlorohydrin in Aqueous Solution. A Sustainable Recycling Strategy in Textile Dyeing Process. Polymers 2019, 11, 252.
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70.
- Eli, W.; Chen, W.; Xue, Q. The association of anionic surfactants with β -cyclodextrin. An isothermal titration calorimeter study. J. Chem. Thermodyn. 1999, 31, 1283–1296.
- Kekes, T.; Tzia, C. Adsorption of indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372.
- Teng, M.; Li, F.; Zhang, B.; Taha, A.A. Electrospun cyclodextrin-functionalized mesoporous polyvinyl alcohol/SiO2 nanofiber membranes as a highly efficient adsorbent for indigo carmine dye. Colloids Surf. A: Physicochem Eng. Asp. 2011, 385, 229–234.
- Chen, P.-Y.; Tung, S.-H. One-Step Electrospinning to Produce Nonsolvent-Induced Macroporous Fibers with Ultrahigh Oil Adsorption Capability. Macromolecules 2017, 50, 2528–2534.
- Liu, J.; Liu, G.; Liu, W. Preparation of water-soluble β-cyclodextrin/poly(acrylic acid)/graphene oxide nanocomposites as new adsorbents to remove cationic dyes from aqueous solutions. Chem. Eng. J. 2014, 257, 299–308.
- Akinyeye, O.J.; Ibigbami, T.B.; Odeja, O. Effect of Chitosan Powder Prepared from Snail Shells to Remove Lead (II) Ion and Nickel (II) Ion from Aqueous Solution and Its Adsorption Isotherm Model. Am. J. Appl. Chem. 2016, 4, 146.
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Nitrate removal from aqueous solutions by cross-linked chitosan beads conditioned with sodium bisulfate. J. Hazard. Mater. 2009, 166, 508–513.
- Cestari, A.R.; Vieira, E.F.S.; Mota, J.A. The removal of an anionic red dye from aqueous solutions using chitosan beads—The role of experimental factors on adsorption using a full factorial design. J. Hazard. Mater. 2008, 160, 337–343.
- Zhao, J.; Zou, Z.; Ren, R.; Sui, X.; Mao, Z.; Xu, H.; Zhong, Y.; Zhang, L.; Wang, B. Chitosan adsorbent reinforced with citric acid modified β-cyclodextrin for highly efficient removal of dyes from reactive dyeing effluents. Eur. Polym. J. 2018, 108, 212–218.
- Krause, R.W.M.; Mamba, B.B.; Bambo, F.M.; Malefetse, T.J. Cyclodextrin polymers: Synthesis and Application in Water Treatment. In Cyclodextrins: Chemistry and Physics; Transworld Research Network: Kerala, India, 2010; ISBN 978-81-7895-430-1.
- Vahedi, S.; Tavakoli, O.; Khoobi, M.; Ansari, A.; Faramarzi, M.A. Application of novel magnetic β-cyclodextrin-anhydride polymer nano-adsorbent in cationic dye removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2017, 80, 452–463.
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Guernelli, S.; Noto, R.; Riela, S. Synthesis and Characterization of Halloysite–Cyclodextrin Nanosponges for Enhanced Dyes Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 3346–3352.
- Zhou, Y.; Hu, Y.; Huang, W.; Cheng, G.; Cui, C.; Lu, J. A novel amphoteric β-cyclodextrin-based adsorbent for simultaneous removal of cationic/anionic dyes and bisphenol A. Chem. Eng. J. 2018, 341, 47–57.
