Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The temperature of a solid tumor is often dissimilar to baseline body temperature and, compared to healthy tissues, may be elevated, reduced, or a mix of both. The temperature of a tumor is dependent on metabolic activity and vascularization and can change due to tumor progression, treatment, or cancer type.
- cancer
- solid tumor
- temperature
- oncolytic virus
1. Introduction
Solid tumors generate heat due to increased vascularization and metabolic activity [1][2]. This provides many tumors with a distinctive elevated temperature compared to surrounding healthy tissues and forms the rationale for detection and diagnosis of breast cancers using thermal imaging [1][3][4]. In contrast, necrotic regions of tumors and certain tumor types, including primary glial tumors and soft tissue lipomatous tumors, are generally cooler than surrounding healthy tissue [5][6]. Tumor temperatures below 37 °C often occur as a byproduct of reduced tumor metabolic activity and/or insufficient tumor vasculature [5]. Despite being a core aspect of the cancer phenotype, cancer research has largely ignored the importance of temperature.
Oncolytic viruses (OVs) are a promising class of cancer therapeutics with the ability to preferentially target and kill tumor cells through a multimodal mechanism of action, leaving healthy tissues relatively unaffected [7]. These mechanisms include direct oncolysis via virus replication, the expression of therapeutic transgenes, the induction of antitumor immune responses via in situ vaccination, starvation of tumor cells via vascular shutdown, and the lethal cell–cell fusion of infected tumor cells [7][8][9][10][11]. Despite the need for OVs to perform optimally at temperatures above and below 37 °C in solid tumors, OVs are almost exclusively tested and optimized at 37 °C. Whereas the effect of elevated temperatures on OVs is relatively understudied, it appears that some OVs may be enhanced and others hindered by elevated temperatures [12][13][14][15][16][17]. This potential divergence in functionality at elevated temperatures is compounded, as OV therapy often generates fevers in patients, further increasing the temperature at which OVs may need to perform [18]. Although a similar divergence of OV functionality at lower temperatures is possible, the absence of research in this area makes speculation difficult. The differential effect of temperature on OVs has major implications with respect to optimization of OV therapies for clinical use. Furthermore, current animal models used in cancer research poorly recapitulate the temperature profile of humans; mice are unable to generate fevers, whereas dogs, cats, and non-human primates have higher baseline body temperatures than humans [19][20][21][22][23]. Overall, a significant refocusing of the cancer and OV fields on understanding the role of temperature is needed.
2. Tumors and Temperature
The heat-generating capacity of tumors is a dynamic variable, changing throughout their progression. Two key parameters work in concert to determine the heat-generating capacity of a tumor: tumor cell metabolism generating heat as a byproduct and vascularization within and around a tumor, which supplies the oxygen and nutrients required for tumor cell proliferation [1][2]. In the early stages of tumor development, a lack of vasculature limits tumor cell metabolism, tumor growth, and, ultimately, heat generation [24][25]. For example, Gimbrone et al. demonstrated that without access to a sufficient blood supply, the growth of a tumor is limited to 1–2 mm3 in diameter [25]. In this situation, a lack of sufficient oxygen causes tumor hypoxia, acting as an angiogenic switch to initiate the formation of new blood vessels [26]. This process, like the act of releasing a foot off the brake pedal, sparks exponential tumor growth, with maximal heat generated at the same time. In later stages of progression, tumors may experience a reduction in heat generation due to the development of necrosis. The longstanding belief as to the development of necrosis is that a tumor outgrows its blood supply due to an imbalance of metabolic activity and vascularization (Figure 1). In contrast, Markwell et al. argues that this late-stage tumor necrosis occurs naturally because of intravascular thrombosis, likely initiated by the overexpression of pro-coagulants by tumor cells [27]. The shutdown of tumor blood vessels then leads to local hypoxia, necrosis, and reduced tumor cell metabolic activity, which are associated with reduced tumor temperature. Subsequently, tumors may again experience localized increases in temperature as the development of hypoxia and necrosis restructure the tumor microenvironment (TME), favoring invasiveness and accelerated tumor growth [27]. As demonstrated for some glial tumors, the heterogeneity of metabolically active tumor cells and regions of necrosis can result in tumors with a marble-like thermal phenotype, with regions of higher and lower temperatures [28]. As identified by Li et al., the hypoxic area surrounding regions of necrosis in glial tumors contains brain tumor stem cells [29]. Overall, the temperature of a tumor varies during its progression and is ultimately a byproduct of vascularization and high metabolic activity.
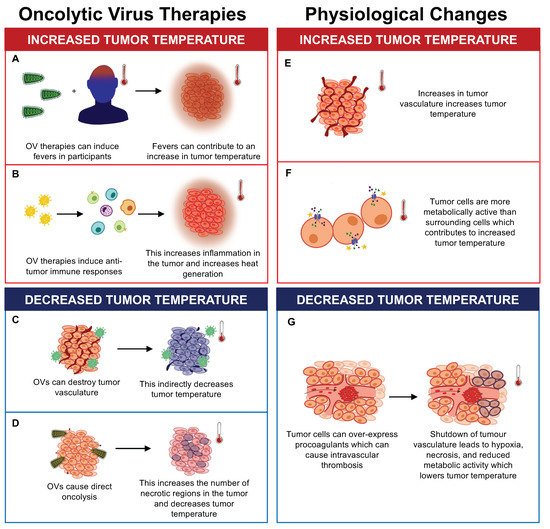
Figure 1. The effects of oncolytic virus (OV) therapy and physiological changes on tumor temperature. OV therapy can increase tumor temperature by (A) causing fevers in recipients and (B) inducing antitumor immune responses, which increase inflammation and heat generation. OV therapy can also decrease tumor temperature by (C) destroying tumor vasculature and (D) causing direct oncolysis of tumor cells, which increases the number of necrotic regions. Physiological changes can also impact tumor temperature. Physiological changes such as (E) increased tumor vasculature and (F) increased metabolic activity of tumor cells can contribute to increases in tumor temperature. (G) Tumor cells have also been shown to overexpress procoagulants which can cause intravascular thrombosis, decreasing tumor temperature due to hypoxia, necrosis, and reduced metabolic activity of tumor cells.
The heat-generating characteristics of tumors have led to the use of thermal imaging for the detection and diagnosis of cancers, particularly breast cancers [1][3][4]. Elevated temperatures have been demonstrated for a handful of cancer types, including those of the breast, bladder, lung, skin, and brain [1][3][4][5][28][30][31][32]. Studies examining the temperature of lung and bladder cancers revealed an average elevated temperature of ~1 °C compared to surrounding normal tissues [30][31]. Interestingly, Yahara et al. determined the average temperature of breast tumors to be 1.79 ± 0.88 °C higher than that of the surrounding tissue [1]. A similar difference was reported by Zhao et al., who identified an average of 1.33 °C elevation in breast tumor temperatures in comparison to patient armpit temperature. This equates to an absolute tumor temperatures ranging from 37.17 to 38.44 °C [33]. The importance of vasculature in determining tumor temperature cannot be understated, as studies have found that increased blood flow and microvessel density correlated with tumor temperatures [1][30][31]. Elevated temperatures have also been detected for metastatic brain tumors [5][28][34]. For example, in a case study examining a patient with metastatic intracortical melanoma, Kateb et al. found an average temperature difference of 1.7 °C [5]. The absolute cortical temperatures recorded for the tumor and healthy brain tissues ranged from 33.5 to 36.5 °C and 33.1 to 33.5 °C, respectively. During surgery, the surface of the brain is ~4 °C cooler than the normal physiological temperature (37 °C) because of the lower operating room temperature (19–20 °C) [5]. Therefore, the actual temperature range of some metastatic brain tumors may be closer to 37.5–40.5 °C, which suggests an overlap with fever-range temperatures (38–41 °C) and hyperthermic treatment (38–45 °C) used in the clinic [35].
Although tumors tend to be elevated in temperature, certain tumors and tumor types have been associated with reduced temperatures. In a study measuring the cortical tumor temperature of six patients with brain tumors (two with metastatic tumors and four with astrocytomas), Koga et al. identified an average temperature reduction of ~2.0 °C compared to surrounding healthy tissues [36]. This corresponded to absolute temperature ranges of 31.1–35.6 °C and 33.0–36.6 °C for tumors and healthy cortex, respectively. Several studies have identified that primary brain tumors of glial origin tend to be hypothermic or lower in temperature than the surrounding healthy tissues [5][28][34]. These studies provide evidence for a distinction between hypothermic primary brain tumors and hyperthermic metastatic brain tumors. Kateb et al. suggested that factors contributing to the lower temperature of primary brain tumors may include a low density of tumor microvessels, lower metabolism in the area surrounding the tumor, greater cerebral spinal fluid in the surrounding tissue, and tumor necrosis [5]. In addition to brain tumors, soft tissue tumors, such as lipomas and atypical lipomatous tumors, have the potential for reduced temperatures [6]. In a thermographic study of soft tissue tumors, Shimatani et al. identified a slight reduction in temperature (0.05 ± 0.17 °C) of the skin located superficial to tumors in 30% (30/100) of patients. Although not a direct measure of core tumor temperature, these findings raise the possibility of reduced temperatures for soft tissue tumors. Shimatani et al. proposed that poor tumor blood flow may be one of the factors contributing to the lower temperatures they identified, based on a previous study demonstrating that lipoma tumors have lower internal vascular flow [6][37]. These studies support the role of reduced vasculature, metabolism, and the presence of tumor necrosis in determining lower tumor temperatures.
The temperature of a tumor can also be affected by therapeutic treatments, as some treatments can generate large numbers of necrotic tumor cells through direct killing or through vascular disruption, thus decreasing the overall metabolic activity and heat generation of a tumor [38]. Tepper et al. showed that following treatment, the temperature of DA3 murine mammary carcinomas was reduced and correlated with core regions of necrosis [38]. In contrast, treatments that cause patients to experience systemic fevers, such as OV-based immunotherapies, are expected to cause transient increases in tumor temperature [18]. No studies have reported the temperature of a patient’s tumor following OV administration. However, researchers postulate that the temperature of a patient’s tumor would increase proportional to the intensity of the fever they experience. This suggests that the temperature an OV faces would vary depending on the baseline temperature of the tumor and the intensity of a patient’s fever. In addition to fever, OV treatment often results in the generation of a robust antitumor immune response accompanied by an influx of highly metabolically active and proinflammatory leukocytes, potentially contributing to enhanced heat generation in a tumor [39]. Therefore, in the case of treatment by OVs, fever and the resulting inflammatory profile of a tumor may contribute to a transient increase in temperature.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10082024
References
- Yahara, T.; Koga, T.; Yoshida, S.; Nakagawa, S.; Deguchi, H.; Shirouzo, K. Relationship between Microvessel Density and Thermographic Hot Areas in Breast Cancer. Surg. Today 2003, 33, 243–248.
- Owens, A.; Godbole, M.; Dabydeen, D.; Medeiros, L.; Phatak, P.; Kandlikar, S. A comparative analysis of the tumor pathology and the metabolic heat generation of growing malignant tumors. In Proceedings of the ASME 2020 18th International Conference on Nanochannels, Microchannels, and Minichannels collocated with the ASME 2020 Heat Transfer Summer Conference and the ASME 2020 Fluids Engineering Division Summer Meeting, Online, 13–15 July 2020.
- Sella, T.; Sklair-Levy, M.; Cohen, M.; Rozin, M.; Shapiro-Feinberg, M.; Allweis, T.M.; Libson, E.; Izhaky, D. A Novel Functional Infrared Imaging System Coupled with Multiparametric Computerised Analysis for Risk Assessment of Breast Cancer. Eur. Radiol. 2013, 23, 1191–1198.
- Arora, N.; Martins, D.; Ruggerio, D.; Tousimis, E.; Swistel, A.J.; Osborne, M.P.; Simmons, R.M. Effectiveness of a Noninvasive Digital Infrared Thermal Imaging System in the Detection of Breast Cancer. Am. J. Surg. 2008, 196, 523–526.
- Kateb, B.; Yamamoto, V.; Yu, C.; Grundfest, W.; Gruen, J.P. Infrared Thermal Imaging: A Review of the Literature and Case Report. Neuroimage 2009, 47, T154–T162.
- Shimatani, A.; Hoshi, M.; Oebisu, N.; Iwai, T.; Takada, N.; Nakamura, H. Clinical Significance of Thermal Detection of Soft-Tissue Tumors. Int. J. Clin. Oncol. 2020, 25, 1418–1424.
- Goradel, N.H.; Baker, A.T.; Arashkia, A.; Ebrahimi, N.; Ghorghanlu, S.; Negahdari, B. Oncolytic Virotherapy: Challenges and Solutions. Curr. Probl. Cancer 2021, 45, 100639.
- Lemos de Matos, A.; Franco, L.S.; McFadden, G. Oncolytic Viruses and the Immune System: The Dynamic Duo. Mol. Ther. Methods Clin. Dev. 2020, 17, 349–358.
- Van Vloten, J.P.; Workenhe, S.T.; Wootton, S.K.; Mossman, K.L.; Bridle, B.W. Critical Interactions between Immunogenic Cancer Cell Death, Oncolytic Viruses, and the Immune System Define the Rational Design of Combination Immunotherapies. J. Immunol. 2018, 200, 450–458.
- Breitbach, C.J.; De Silva, N.S.; Falls, T.J.; Aladl, U.; Evgin, L.; Paterson, J.; Sun, Y.Y.; Roy, D.G.; Rintoul, J.L.; Daneshmand, M.; et al. Targeting Tumor Vasculature with an Oncolytic Virus. Mol. Ther. 2011, 19, 886–894.
- Krabbe, T.; Altomonte, J. Fusogenic Viruses in Oncolytic Immunotherapy. Cancers 2018, 10, 216.
- Jung, B.K.; Lee, Y.K.; Hong, J.; Ghandehari, H.; Yun, C.O. Mild Hyperthermia Induced by Gold Nanorod-Mediated Plasmonic Photothermal Therapy Enhances Transduction and Replication of Oncolytic Adenoviral Gene Delivery. ACS Nano 2016, 10, 10533–10543.
- Eisenberg, D.P.; Carpenter, S.G.; Adusumilli, P.S.; Chan, M.K.; Hendershott, K.J.; Yu, Z.; Fong, Y. Hyperthermia Potentiates Oncolytic Herpes Viral Killing of Pancreatic Cancer through a Heat Shock Protein Pathway. Surgery 2010, 148, 325–334.
- Chang, E.; Chalikonda, S.; Friedl, J.; Xu, H.; Phan, G.Q.; Marincola, F.M.; Alexander, H.R.; Bartlett, D.L. Targeting Vaccinia to Solid Tumors with Local Hyperthermia. Hum. Gene Ther. 2005, 16, 435–444.
- Vasconcelos, D.Y.; Cai, X.H.; Oglesbee, M.J. Constitutive Overexpression of the Major Inducible 70 KDa Heat Shock Protein Mediates Large Plaque Formation by Measles Virus. J. Gen. Virol. 1998, 79, 2239–2247.
- De Marco, A.; Santoro, M.G. Antiviral Effect of Short Hyperthermic Treatment at Specific Stages of Vesicular Stomatitis Virus Replication Cycle. J. Gen. Virol. 1993, 74, 1685–1690.
- Schirrmacher, V.; Bihari, A.S.; Stücker, W.; Sprenger, T. Long-Term Remission of Prostate Cancer with Extensive Bone Metastases upon Immuno- and Virotherapy: A Case Report. Oncol. Lett. 2014, 8, 2403–2406.
- Fu, L.Q.; Wang, S.B.; Cai, M.H.; Wang, X.J.; Chen, J.Y.; Tong, X.M.; Chen, X.Y.; Mou, X.Z. Recent Advances in Oncolytic Virus-Based Cancer Therapy. Virus Res. 2019, 270, 197675.
- Hankenson, F.C.; Ruskoski, N.; Van Saun, M.; Ying, G.S.; Oh, J.; Fraser, N.W. Weight Loss and Reduced Body Temperature Determine Humane Endpoints in a Mouse Model of Ocular Herpesvirus Infection. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 277–285.
- Austgen, L.E.; Bowen, R.A.; Bunning, M.L.; Davis, B.S.; Mitchell, C.J.; Chang, G.J.J. Experimental Infection of Cats and Dogs with West Nile Virus. Emerg. Infect. Dis. 2004, 10, 82–86.
- Goic, J.B.; Reineke, E.L.; Drobatz, K.J. Comparison of Rectal and Axillary Temperatures in Dogs and Cats. J. Am. Vet. Med. Assoc. 2014, 244, 1170–1175.
- Pol, J.G.; Acuna, S.A.; Yadollahi, B.; Tang, N.; Stephenson, K.B.; Atherton, M.J.; Hanwell, D.; El-Warrak, A.; Goldstein, A.; Moloo, B.; et al. Preclinical Evaluation of a MAGE-A3 Vaccination Utilizing the Oncolytic Maraba Virus Currently in First-in-Human Trials. Oncoimmunology 2019, 8, e1512329.
- Li, X.F.; Dong, H.L.; Huang, X.Y.; Qiu, Y.F.; Wang, H.J.; Deng, Y.Q.; Zhang, N.N.; Ye, Q.; Zhao, H.; Liu, Z.Y.; et al. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-Human Primates. EBioMedicine 2016, 12, 170–177.
- Ruoslahti, E. Specialization of Tumour Vasculature. Nat. Rev. Cancer 2002, 2, 83–90.
- Gimbrone, M.A.; Leapman, S.B.; Cotran, R.S.; Folkman, J. Tumor Dormancy in Vivo by Prevention of Neovascularization. J. Exp. Med. 1972, 136, 261–276.
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor Angiogenesis: Causes, Consequences, Challenges and Opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770.
- Markwell, S.M.; Ross, J.L.; Olson, C.L.; Brat, D.J. Necrotic Reshaping of the Glioma Microenvironment Drives Disease Progression. Acta Neuropathol. 2022, 143, 291–310.
- Gorbach, A.M.; Heiss, J.D.; Kopylev, L.; Oldfield, E.H. Intraoperative Infrared Imaging of Brain Tumors. J. Neurosurg. 2004, 101, 960–969.
- Li, Z.; Bao, S.; Wu, Q.; Wang, H.; Eyler, C.; Sathornsumetee, S.; Shi, Q.; Cao, Y.; Lathia, J.; McLendon, R.E.; et al. Hypoxia-Inducible Factors Regulate Tumorigenic Capacity of Glioma Stem Cells. Cancer Cell 2009, 15, 501–513.
- Stefanadis, C.; Chrysochoou, C.; Markou, D.; Petraki, K.; Panagiotakos, D.B.; Fasoulakis, C.; Kyriakidis, A.; Papadimitriou, C.; Toutouzas, P.K. Increased Temperature of Malignant Urinary Bladder Tumors In Vivo: The Application of a New Method Based on a Catheter Technique. J. Clin. Oncol. 2001, 19, 676–681.
- Stefanadis, C.; Chrysohoou, C.; Panagiotakos, D.B.; Passalidou, E.; Katsi, V.; Polychronopoulos, V.; Toutouzas, P.K. Temperature Differences Are Associated with Malignancy on Lung Lesions: A Clinical Study. BMC Cancer 2003, 3, 1.
- Pirtiniçetingl, M.; Herman, C. Quantification of the Thermal Signature of a Melanoma Lesion. Int. J. Therm. Sci. 2011, 50, 421–431.
- Zhao, Q.; Zhang, J.; Wang, R.; Cong, W. Use of a Thermocouple for Malignant Tumor Detection. IEEE Eng. Med. Biol. Mag. 2008, 27, 64–66.
- Ecker, R.D.; Goerss, S.J.; Meyer, F.B.; Cohen-Gadol, A.A.; Britton, J.W.; Levine, J.A. Vision of the Future: Initial Experience with Intraoperative Real-Time High-Resolution Dynamic Infrared Imaging: Technical Note. J. Neurosurg. 2002, 97, 1460–1471.
- Repasky, E.A.; Evans, S.S.; Dewhirst, M.W. Temperature Matters! And Why It Should Matter to Tumor Immunologists. Cancer Immunol. Res. 2013, 1, 210–216.
- Koga, H.; Mori, K.; Ono, H.; Kuwahara, M.; Matsuse, E. Intraoperative Regional Thermography during Surgery for Brain Tumors. Neurol. Med. Chir. 1987, 27, 1033–1038.
- Szymańska, A.; Szymański, M.; Gołąbek, W.; Drelich-Zbroja, A.; Jargiełło, T. Doppler Ultrasound Appearance of Neck Tumors. J. Ultrason. 2018, 18, 96–102.
- Tepper, M.; Shoval, A.; Hoffer, O.; Confino, H.; Schmidt, M.; Kelson, I.; Keisari, Y.; Gannot, I. Thermographic Investigation of Tumor Size, and Its Correlation to Tumor Relative Temperature, in Mice with Transplantable Solid Breast Carcinoma. J. Biomed. Opt. 2013, 18, 111410.
- Allison, K.E.; Coomber, B.L.; Bridle, B.W. Metabolic Reprogramming in the Tumour Microenvironment: A Hallmark Shared by Cancer Cells and T Lymphocytes. Immunology 2017, 152, 175–184.
This entry is offline, you can click here to edit this entry!
