Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Differential diagnosis between Blastic pDC Neoplasm (BPDCN) and Acute Myeloid Leukemia with pDC expansion (pDC-AML) is particularly challenging, and genomic features can help in diagnosis. The genetic landscape of BPDCN is now well-defined, with important updates concerning MYC/MYC rearrangements, but also epigenetic defects and novel concepts in oncogenic and immune pathways.
- mature plasmacytoid dendritic cells proliferation
- acute myeloid leukemia
- RUNX1 mutation
1. Genetics of Blastic Plasmacytoid Dendritic Cell Neoplasms
Cytogenetic abnormalities are detected in 57 to 75% of BPDCN patients. Most of the karyotypes contain a wide spectrum of cytogenetic abnormalities, leading to a complex karyotype (CK) (≥3 aberrations) in more than 50% of cases [1][2]. Abnormal karyotypes of BPDCN show a high number of aberrations (mean = 6.8 per case). Using conventional cytogenetic and Fluorescent In Situ Hybridization (FISH)/multi-FISH approaches, a special and distinct cytogenetic signature of BPDCN have been described, showing various but recurrent chromosomal losses or deletions over gains. These abnormalities include 6 major recurrent chromosomal losses detected at high frequency among abnormal karyotype: 5q deletion (72%), 12p deletion (64%), 13q deletion or monosomy 13 (64%), 6q deletion (50%), 15q deletion or monosomy 15 (43%), and monosomy 9 (28%) [1]. Remarkably, among CK, careful examination revealed that three or more of these six chromosomal targets were associated in 50% of cases, defining a special cytogenetic signature for BPDCN. These results have been confirmed by two independent studies [3][4].
These recurrent deletions were confirmed by chromosomal microarrays analyses, with losses of 9p21.3 (CDKN2A/CDKN2B), 12p13.2-p13.1 (CDKN1B, ETV6), 13q11-q21 (LATS2, RB1), 5q31 (NR3C1), or 7p12.2 (IKZF1) [1][2][5]. Despite this original pattern of recurrent abnormalities, there is no unique key genetic event in BPDCN. Indeed, at least four genes are particularly deleted and/or mutated: IKZF1, required for BPDCN differentiation [6]; RB1, potentially associated with transformation in the case of biallelic inactivation [7], ETV6, whose deletion would correspond to an early pathogenic event [4], and NR3C1, involved in the glucocorticoid metabolism [3]. The transcriptional activators MYC and MYB are also originally rearranged at high frequency in this neoplasm. Remarkably, the significant association of these alterations (i.e., loss of CDKN2A-B/9p21, CDKN1B/12p13, or RB1/13q14, rearrangement of MYC/8q24 or MYB/6q23) constitute the special and unique pattern frequently detected in BPDCN.
2. Genetics of Blastic Plasmacytoid Dendritic Cell Neoplasms
2.1. Deletions Involving Immune Genes
Interestingly, these recurrent deletions also involve genes related to the original function of pDC, i.e., immune response, especially losses of 6q23 (IFNGR1, TNFAIP3), 9p21.3 (cluster of IFNA genes), and 12p13.2-p13.1 (CLEC2B, CLEC4C, CLEC4E, TNFRSF1A) [8]. Thus, those deletions compromise the normal function of the cells of origin of BPDCN.
2.2. Inactivation of Genes Encoding Cell-Cycle Inhibitors and Tumor Suppressor Genes
Similarly to other hematological malignancies, deletions inactivating Tumor Suppressor Genes (TSG), such as TP53 [9][10][11][12], responsible for genetic instability, are also found in BPDCN. The tumor suppressor gene ATM, mutated in lymphoproliferative syndromes [13], may also be mutated in BPDCN [12], while RB1, involved in the regulation of the G1/S cell cycle transition, is also frequently deleted [5][10][12][14]. Initially described in retinoblastoma, in which it modeled the principle of TSG [15], RB1 is also reported in chronic lymphocytic leukemia and Acute Lymphoblastic Leukemia (ALL) [16]. Additionally, the CDKN1B/CDKN2B/CDKN2A genes have a role in the G1/S transition, and their deletions are reported in some studies [2][5][10][12][17], similar to ALL [16] and lymphoma [18]. This alteration in cell cycle regulation could have a crucial role in the oncogenesis of BPDCN [10].
2.3. Recurrent Deletions in 5q31
Deletion in 5q are particularly recurrent in BPDCN, constituting a specific defect compared to other hematological malignancies [3][19]. In the 5q23.3 Common Deleted Region (CDR), HINT1 was first proposed to be a key gene [19]. Indeed, HINT1 encodes a homodimeric purine phosphoramidase, suggesting a transcriptional modulatory role. Moreover, HINT1 deficiency would impair ATM function and thus DNA repair [19]. On the other hand, the 5q31 locus would also be a key region on chromosome 5, with deletions delineating a group of unfavorable prognostic impacts [3]. The glucocorticoid receptor gene NR3C1 was found to be recurrently deleted, leading to haploinsufficiency and decreased glucocorticoid receptor activity [3]. The deletions impact the polycomb complex, in particular EZH2, with dysregulation of the HOXA locus and plasmacytoid dendritic differentiation.
2.4. Deletions of Transcription Factors
Similarly to other hematological neoplasms, transcription factor are particularly impacted in BPDCN. ETV6 (TEL) is frequently mutated or deleted [1][5][8][9][10][17], contrasting with classical defects in other leukemia where translocations are more frequent, including t(12;21)(p13;q22) ETV6::RUNX1 in B-cell Acute Lymphoblastic Leukemia (B-ALL) [20], t(5;12)(q32;p13) ETV6::PDGFRB, t(9;12)(p24;p13) ETV6::JAK2 or t(4;12) (q12;p13) ETV6::PDGFRA in hypereosinophilic syndromes, and other translocations in rare cases of Acute Myeloid Leukemia (AML) [21]. ETV6 invalidations are, however, known in leukemia: somatic mutations of ETV6 remain rare in AML [21], but germline mutations are also possible in the context of thrombocytopenia predisposing to AML, Myelodysplastic Syndromes (MDS), Chronic MyeloMonocytic Leukemia (CMML), B-ALL, or multiple myeloma [22].
The IKAROS family (IKZF1/2/3) is also frequently deleted [2][9][14][23], similarly to ALL [24], where it compromises lymphoid differentiation [25]. ZEB2 may also be altered in BPDCN [26]. This transcription factor is involved in the commitment and lineage fidelity of myeloid and lymphoid cells at various stages of hematopoiesis and is thought to play a key role in the development of various types of AML, ALL, and lymphoma [27].
While translocations involving transcription factors have been widely described in ALL and AML, recurrent rearrangements in BPDCN were rare before 2017. Indeed, KMT2A (MLL) rearrangements had previously been described in rare cases of CD4+ CD56+ neoplams identified as BPDCN (KMT2A::ENL and KMT2A::MLLT1) [28][29], but these descriptions in BDPCN have been challenged because these reported cases do not fulfill the current diagnostic criteria of BPDCN. Indeed, these cases could correspond to CD4+ CD56+ AML, because they constitute a delicate differential diagnosis for BPDCN [30].
2.5. Recurrent MYC Rearrangements
Initially, the translocation t(6;8)(p21;q24) was sporadically reported by several studies, using conventional karyotyping. Since 2018, MYC rearrangements (8q24) have been largely described in approximately 30% of BPDCN [23][31][32][33], with a more frequent immunoblastoid morphology and sometimes a CD56-negative phenotype [31][34].
These MYC abnormalities bring BPDCN closer to high-grade B lymphoma [2][6][31][35], but the gene partners are really different, with specific partners that could point towards pDC differentiation. Indeed, among MYC rearranged cases of BPDCN, Sakamoto et al. confirmed the high prevalence of the t(6;8)—detected in 56% of cases involving the RUNX2 locus at 6p21. Interestingly, Kubota et al. showed that the t(6;8) juxtaposes the promoter of MYC to the pDCs-specific RUNX2 super-enhancer, leading to overexpression of MYC. In this recurrent t(6;8)(p21;q24), both MYC and RUNX2 are dysregulated, and cooperate together to promote survival and proliferation of the BPDCN cells. Remarkably, RUNX2 is physiologically involved in differentiation and migration of pDCs and plays a dominant role in controlling transcription networks in BPDCN [36].
Other partners of MYC have been sporadically reported but not clearly identified (i.e., 2p12, Xq24, 3p25, 14q32). It remains to be determined if MYC rearrangement could constitute a primary or secondary genetic event in BPDCN. In this way, the t(6;8)(p21;q24) cannot be considered as a specific genetic abnormality of BPDCN because it has been reported in follicular lymphoma [37]. Lastly, a unique study showed the adverse impact of MYC rearrangement, and this prognostic impact still needs to be confirmed by further independent studies [31].
2.6. Recurrent MYB Rearrangements
In 2017, other recurrent rearrangements were described in nine of fourteen patients, including five children [14]. Remarkably, all five children included in this series had a MYB rearrangement. Of note, the previous largest report of pediatric BPDCN cases exhibited several cases with 1q and/or 6q abnormalities, or translocation t(1;6)(q21;q23) [38]. These observations reveal a striking link between pediatric BPDCN and MYB rearrangement.
MYB rearrangements create fusion transcripts between MYB and various partner genes (ZFAT/8q24, PLEKHO1/1q21, DCPS/11q24, miR-3134/3p25) [14]. The chimeric transcripts retain the MYB transactivation domain and disrupt its negative regulatory domain, which allows the maintenance of the MYB transcriptional activity. Indeed, functional analysis of MYB fusions revealed the activation of MYB target genes as a result of induced MYB activation [14]. MYB is a nuclear-localized transcriptional activator in hematopoietic cells that interacts with the C/EBP complex to stimulate the transcriptional activity of MYC, BCL2, c-KIT, c-ERBB2, and other targets (Figure 1). Its expression progressively decreases during cell differentiation, with high activity in hematopoietic stem cells and activated T-cells.
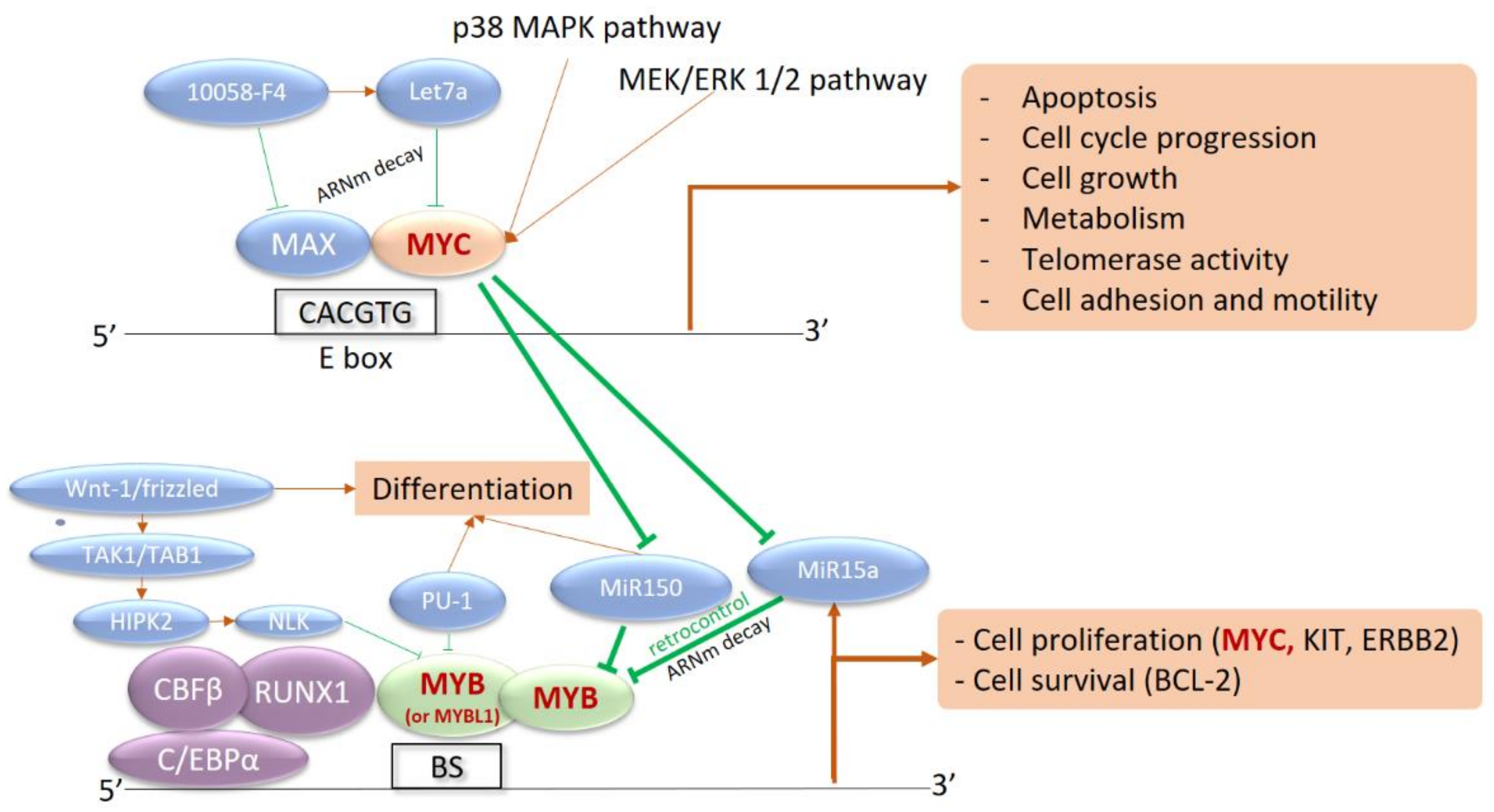
Figure 1. Interaction network between MYB, MYC, and their transcriptional targets. MYC is a strong transcriptional activator, dependent on the intracellular phosphorylation cascade signaling pathways of Mitogen-Activated Protein Kinases (MAPK) and Extracellular signal-Regulated Kinases (ERK) (p38 MAPK and MEK/ERK pathways 1 and 2). Activation of MYC induces the transcription of numerous target genes involved in proliferation, division, metabolism, and cell motility, as well as apoptosis. MYC also inhibits microRNAs (MiR150 and MiR15a) that are capable of silencing MYB expression in the basal state. Activation of MYC therefore induces activation of MYB, involved in the core binding factor (CBF) complex with CBFB, RUNX1, and CEBPA. MYB is also a transcriptional activator recognizing multiple nucleotide sequences, in a complex with CEBP. The targeted genes are involved in survival with BCL-2 and cell proliferation with c-KIT, c-ERBB2, and especially MYC. This results in an activation loop between the two transcriptional activators: BS, MYB Binding Site with MYB Recognition Element.
In contrast to MYC, MYB is only exceptionally rearranged in other hematological malignancies: in fact, only in rare cases of acute basophilic leukemia with MYB::GATA1 fusion transcripts, even rarer than BPDCN [39] and in T-ALL with t(6;7) and MYB duplication [40]. MYB translocations are also reported in 60–80% of adenoid cystic carcinomas, mainly with the MYB::NFIB fusion transcript [41][42], and in pediatric gliomas [43]. For the first time, fusion transcripts appear to be recurrent and specific for BPDCN compared to other hematological malignancies. MYB may play a key role in the leukemic transformation process, similar to MYB::GATA1 rearrangements in acute basophilic leukemia. However, the frequency of MYB rearrangements is very uncertain and possibly higher in young patients, as suggested by the original study [14].
Interestingly, an analog of MYB, MYBL1 would also be rearranged in a very similar way, with an identical functional impact [31]. Finally, MYB, MYBL1, and MYC defects would be mutually exclusive, delineating distinct groups of patients [31].
2.7. Mutation Landscape
2.7.1. A Myeloid-like Profile
In addition to these cytogenetic defects, the mutation landscape of BPDCN has been particularly studied. However, given the rarity of BPDCN, only small cohorts have been studied so far (less than 30 cases), and usually by targeted high-throughput sequencing. Nevertheless, 22 Whole Exome Sequencing (WES) have been performed [7][9][44]. On a first stratum, the mutation landscape is quite similar to myeloid neoplasms [45][46][47][48][49][50], with a high prevalence of mutations involving epigenetics (TET2, ASXL1) and splicing (ZRSR2, SRSF2, U2AF1) [9][11][51][52][53]. These mutations rather suggest an early process before leukemic transformation, as is well described in AML, disturbing the DNA methylation balance, modifying chromatin access and the splicing processes [54]. In multistage leukemogenesis models, epigenetics and splicing mutations would be present from the pre-leukemic stages [55], and their frequency increases with age. Of note, mutations of TET2 are found in 40 to 60% of cases [9][11][53]. Interestingly, loss-of-function of ZRSR2 impairs pDC activation and apoptosis after inflammatory stimuli with intron retention, promoting pDC expansion. Of note, being located on the X chromosome, this enrichment of ZRSR2 in BPDCN fits well with its predominance in males [52]. Although mutations of NPM1 were initially described in BPDCN [9], this has not been confirmed since, and this is not consistent with the nature of these mutations defining a mutually exclusive subtype of AML [22]. In contrast, sub-clonal mutations of signaling pathways can be found in 5 to 20% of BPDCN, especially FLT3, KIT, KRAS, and NRAS mutations [9][12][14][26][44][45][53][56][57][58]. This profile is close to that of CMML and is consistent with a common clonal origin of BPDCN and CMML cells demonstrated in a few patients suffering from the two neoplasms [7][45]. The leukemic model would include shared epigenetic mutations, with secondary emergence of a BPDCN clone and another clone leading to CMML [48][59] or AML [60][61][62][63].
2.7.2. Some Lymphoid-like Features
Associated with these “myeloid-like”, key deleted transcription factors or tumor suppressor genes IKZF1, ETV6, RB1, ATM, and TP53 can also be mutated in some cases (5–10%), also resulting in an invalidation [2][4][6][7][11][64][65]. Notably, biallelic invalidations of ETV6 argue for a primordial early event, possibly overexpressing the BPDCN oncogene TCL1A [4][8][66]. IKZF1 loss-of-function, either by deletion or mutation, would lead to the increased cell interactions in BPDCN. BPDCN also exhibit KMT2D and SYNE1 mutations or losses, previously reported in follicular lymphoma [6][64]. Overall, the most characteristic feature of BPDCN would be that combination of myeloid-like and lymphoid-like abnormalities (Figure 2).
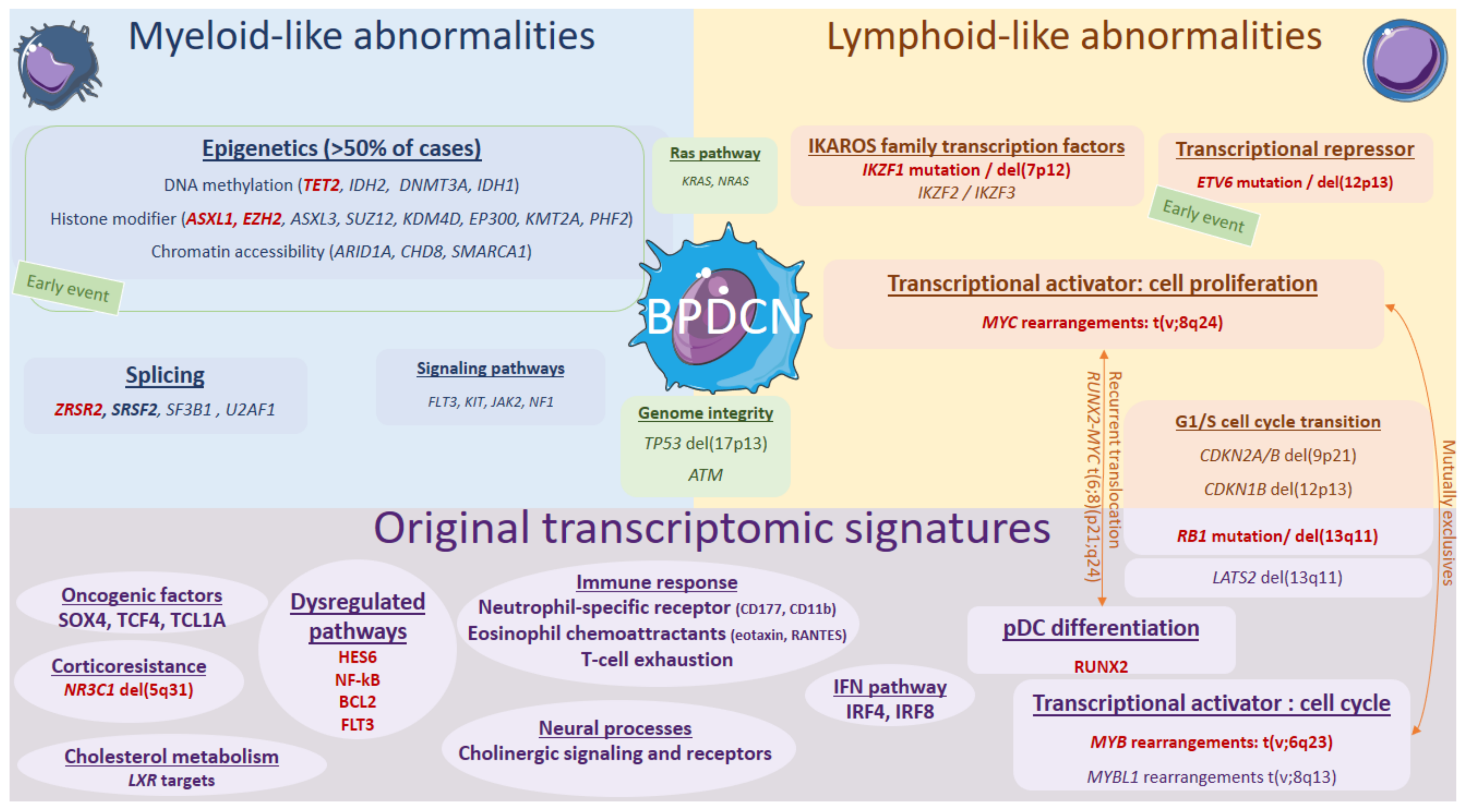
Figure 2. Genomics and transcriptional landscape of BPDCN. The genomic landscape of BPDCN include a combination of myeloid-like and lymphoid-like mutations and cytogenetic defects within a complex landscape, with frequent complex karyotypes. The transcriptional program of BPDCN is made of a diversity of original factors: RUNX2, MYB, IFN pathway, neural processes, cholesterol metabolism, corticoresistance factor, and original oncogenic factors.
2.8. Prognostic Factors
A major challenge to determine the mutation landscape in BPDCN is to establish a molecular prognostic stratification, as in AML with the European Leukemia Network recommendation [67]. Nevertheless, this point remains a tricky issue because of the rarity and diagnostic challenges of BPDCN. Some adverse prognostic factors have still been proposed, particularly mutations involving ETV6, TP53, or NRAS/KRAS [9], as well as biallelic deletions of 9p21.3 [1][2], deletions of NR3C1 [3], abnormal karyotype or numerous abnormalities detected [68][69], and rearrangements of MYC [31][70]. At this time, it is unfortunately impossible to integrate all of these factors into a score stratifying patients, and most of these prognostic abnormalities need to be confirmed.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14174132
References
- Leroux, D.; Mugneret, F.; Callanan, M.; Radford-Weiss, I.; Dastugue, N.; Feuillard, J.; Le Mée, F.; Plessis, G.; Talmant, P.; Gachard, N.; et al. CD4(+), CD56(+) DC2 Acute Leukemia Is Characterized by Recurrent Clonal Chromosomal Changes Affecting 6 Major Targets: A Study of 21 Cases by the Groupe Français de Cytogénétique Hématologique. Blood 2002, 99, 4154–4159.
- Lucioni, M.; Novara, F.; Fiandrino, G.; Riboni, R.; Fanoni, D.; Arra, M.; Venegoni, L.; Nicola, M.; Dallera, E.; Arcaini, L.; et al. Twenty-One Cases of Blastic Plasmacytoid Dendritic Cell Neoplasm: Focus on Biallelic Locus 9p21.3 Deletion. Blood 2011, 118, 4591–4594.
- Emadali, A.; Hoghoughi, N.; Duley, S.; Hajmirza, A.; Verhoeyen, E.; Cosset, F.-L.; Bertrand, P.; Roumier, C.; Roggy, A.; Suchaud-Martin, C.; et al. Haploinsufficiency for NR3C1, the Gene Encoding the Glucocorticoid Receptor, in Blastic Plasmacytoid Dendritic Cell Neoplasms. Blood 2016, 127, 3040–3053.
- Tang, Z.; Li, Y.; Wang, W.; Yin, C.C.; Tang, G.; Aung, P.P.; Hu, S.; Lu, X.; Toruner, G.A.; Medeiros, L.J.; et al. Genomic Aberrations Involving 12p/ETV6 Are Highly Prevalent in Blastic Plasmacytoid Dendritic Cell Neoplasms and Might Represent Early Clonal Events. Leuk. Res. 2018, 73, 86–94.
- Wiesner, T.; Obenauf, A.C.; Cota, C.; Fried, I.; Speicher, M.R.; Cerroni, L. Alterations of the Cell-Cycle Inhibitors P27(KIP1) and P16(INK4a) Are Frequent in Blastic Plasmacytoid Dendritic Cell Neoplasms. J. Investig. Dermatol. 2010, 130, 1152–1157.
- Bastidas Torres, A.N.; Cats, D.; Mei, H.; Fanoni, D.; Gliozzo, J.; Corti, L.; Paulli, M.; Vermeer, M.H.; Willemze, R.; Berti, E.; et al. Whole-Genome Analysis Uncovers Recurrent IKZF1 Inactivation and Aberrant Cell Adhesion in Blastic Plasmacytoid Dendritic Cell Neoplasm. Genes Chromosomes Cancer 2020, 59, 295–308.
- Patnaik, M.M.; Lasho, T.; Howard, M.; Finke, C.; Ketterling, R.L.; Al-Kali, A.; Pardanani, A.; Droin, N.; Gangat, N.; Tefferi, A.; et al. Biallelic Inactivation of the Retinoblastoma Gene Results in Transformation of Chronic Myelomonocytic Leukemia to a Blastic Plasmacytoid Dendritic Cell Neoplasm: Shared Clonal Origins of Two Aggressive Neoplasms. Blood Cancer J. 2018, 8, 82.
- Renosi, F.; Roggy, A.; Giguelay, A.; Soret, L.; Viailly, P.-J.; Cheok, M.; Biichle, S.; Angelot-Delettre, F.; Asnafi, V.; Macintyre, E.; et al. Transcriptomic and Genomic Heterogeneity in Blastic Plasmacytoid Dendritic Cell Neoplasms: From Ontogeny to Oncogenesis. Blood Adv. 2021, 5, 1540–1551.
- Menezes, J.; Acquadro, F.; Wiseman, M.; Gómez-López, G.; Salgado, R.N.; Talavera-Casañas, J.G.; Buño, I.; Cervera, J.V.; Montes-Moreno, S.; Hernández-Rivas, J.M.; et al. Exome Sequencing Reveals Novel and Recurrent Mutations with Clinical Impact in Blastic Plasmacytoid Dendritic Cell Neoplasm. Leukemia 2014, 28, 823–829.
- Jardin, F.; Callanan, M.; Penther, D.; Ruminy, P.; Troussard, X.; Kerckaert, J.P.; Figeac, M.; Parmentier, F.; Rainville, V.; Vaida, I.; et al. Recurrent Genomic Aberrations Combined with Deletions of Various Tumour Suppressor Genes May Deregulate the G1/S Transition in CD4+CD56+ Haematodermic Neoplasms and Contribute to the Aggressiveness of the Disease. Leukemia 2009, 23, 698–707.
- Jardin, F.; Ruminy, P.; Parmentier, F.; Troussard, X.; Vaida, I.; Stamatoullas, A.; Leprêtre, S.; Penther, D.; Duval, A.B.; Picquenot, J.-M.; et al. TET2 and TP53 Mutations Are Frequently Observed in Blastic Plasmacytoid Dendritic Cell Neoplasm. Br. J. Haematol. 2011, 153, 413–416.
- Stenzinger, A.; Endris, V.; Pfarr, N.; Andrulis, M.; Jöhrens, K.; Klauschen, F.; Siebolts, U.; Wolf, T.; Koch, P.-S.; Schulz, M.; et al. Targeted Ultra-Deep Sequencing Reveals Recurrent and Mutually Exclusive Mutations of Cancer Genes in Blastic Plasmacytoid Dendritic Cell Neoplasm. Oncotarget 2014, 5, 6404–6413.
- Gumy-Pause, F.; Wacker, P.; Sappino, A.-P. ATM Gene and Lymphoid Malignancies. Leukemia 2004, 18, 238–242.
- Suzuki, K.; Suzuki, Y.; Hama, A.; Muramatsu, H.; Nakatochi, M.; Gunji, M.; Ichikawa, D.; Hamada, M.; Taniguchi, R.; Kataoka, S.; et al. Recurrent MYB Rearrangement in Blastic Plasmacytoid Dendritic Cell Neoplasm. Leukemia 2017, 31, 1629–1633.
- Dyson, N.J. RB1: A Prototype Tumor Suppressor and an Enigma. Genes Dev. 2016, 30, 1492–1502.
- Mullighan, C.G. The Genomic Landscape of Acute Lymphoblastic Leukemia in Children and Young Adults. Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 174–180.
- Tang, Z.; Tang, G.; Wang, S.A.; Lu, X.; Young, K.H.; Bueso-Ramos, C.E.; Alvarado, Y.; Medeiros, L.J.; Khoury, J.D. Simultaneous Deletion of 3’ETV6 and 5’EWSR1 Genes in Blastic Plasmacytoid Dendritic Cell Neoplasm: Case Report and Literature Review. Mol. Cytogenet. 2016, 9, 23.
- Jardin, F.; Jais, J.-P.; Molina, T.-J.; Parmentier, F.; Picquenot, J.-M.; Ruminy, P.; Tilly, H.; Bastard, C.; Salles, G.-A.; Feugier, P.; et al. Diffuse Large B-Cell Lymphomas with CDKN2A Deletion Have a Distinct Gene Expression Signature and a Poor Prognosis under R-CHOP Treatment: A GELA Study. Blood 2010, 116, 1092–1104.
- Fu, Y.; Fesler, M.; Mahmud, G.; Bernreuter, K.; Jia, D.; Batanian, J.R. Narrowing down the Common Deleted Region of 5q to 6.0 Mb in Blastic Plasmacytoid Dendritic Cell Neoplasms. Cancer Genet. 2013, 206, 293–298.
- Montaño, A.; Ordoñez, J.L.; Alonso-Pérez, V.; Hernández-Sánchez, J.; Santos, S.; González, T.; Benito, R.; García-Tuñón, I.; Hernández-Rivas, J.M. ETV6/RUNX1 Fusion Gene Abrogation Decreases the Oncogenicity of Tumour Cells in a Preclinical Model of Acute Lymphoblastic Leukaemia. Cells 2020, 9, 215.
- Qinrong, W.; Shasha, D.; Hong, Y.; Lijun, W.; Huiying, Q.; Llili, Q.; Liang, M.; Suning, C. ETV6 Mutation in a Cohort of 970 Patients with Hematologic Malignancies. Haematologica 2014, 99, e176.
- Swerdlow, S.H.; Campo, E.; Harris, N.L.; Jaffe, E.S.; Pileri, S.A.; Stein, H.; Thiele, J.; Arber, D.A.; Hasserjian, R.; Le Beau, M.M.; et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 2, ISBN 978-92-832-449-3.
- Tzankov, A.; Hebeda, K.; Kremer, M.; Leguit, R.; Orazi, A.; van der Walt, J.; Gianelli, U. Plasmacytoid Dendritic Cell Proliferations and Neoplasms Involving the Bone Marrow: Summary of the Workshop Cases Submitted to the 18th Meeting of the European Association for Haematopathology (EAHP) Organized by the European Bone Marrow Working Group, Basel 2016. Ann. Hematol. 2017, 96, 765–777.
- Dhanyamraju, P.K.; Iyer, S.; Smink, G.; Bamme, Y.; Bhadauria, P.; Payne, J.L.; Dovat, E.; Klink, M.; Ding, Y. Transcriptional Regulation of Genes by Ikaros Tumor Suppressor in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2020, 21, 1377.
- Chen, Q.; Shi, Y.; Chen, Y.; Ji, T.; Li, Y.; Yu, L. Multiple Functions of Ikaros in Hematological Malignancies, Solid Tumor and Autoimmune Diseases. Gene 2019, 684, 47–52.
- Ladikou, E.; Ottolini, B.; Nawaz, N.; Allchin, R.L.; Payne, D.; Ali, H.; Marafioti, T.; Shaw, J.; Ahearne, M.J.; Wagner, S.D. Clonal Evolution in the Transition from Cutaneous Disease to Acute Leukemia Suggested by Liquid Biopsy in Blastic Plasmacytoid Dendritic Cell Neoplasm. Haematologica 2018, 103, e196–e199.
- Soen, B.; Vandamme, N.; Berx, G.; Schwaller, J.; Van Vlierberghe, P.; Goossens, S. ZEB Proteins in Leukemia: Friends, Foes, or Friendly Foes? HemaSphere 2018, 2, e43.
- Toya, T.; Nishimoto, N.; Koya, J.; Nakagawa, M.; Nakamura, F.; Kandabashi, K.; Yamamoto, G.; Nannya, Y.; Ichikawa, M.; Kurokawa, M. The First Case of Blastic Plasmacytoid Dendritic Cell Neoplasm with MLL-ENL Rearrangement. Leuk. Res. 2012, 36, 117–118.
- Yang, N.; Huh, J.; Chung, W.S.; Cho, M.-S.; Ryu, K.-H.; Chung, H.-S. KMT2A (MLL)-MLLT1 Rearrangement in Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancer Genet. 2015, 208, 464–467.
- Lee, J.M.; Kim, I.S.; Lee, J.N.; Park, S.H.; Kim, H.H.; Chang, C.L.; Lee, E.Y.; Kim, H.R.; Oh, S.H.; Song, S.A. Acute Myeloid Leukemia with MLL Rearrangement and CD4+/CD56+ Expression Can Be Misdiagnosed as Blastic Plasmacytoid Dendritic Cell Neoplasm: Two Case Reports. Ann. Lab. Med. 2016, 36, 494–497.
- Sakamoto, K.; Katayama, R.; Asaka, R.; Sakata, S.; Baba, S.; Nakasone, H.; Koike, S.; Tsuyama, N.; Dobashi, A.; Sasaki, M.; et al. Recurrent 8q24 Rearrangement in Blastic Plasmacytoid Dendritic Cell Neoplasm: Association with Immunoblastoid Cytomorphology, MYC Expression, and Drug Response. Leukemia 2018, 32, 2590–2603.
- Kurt, H.; Khoury, J.D.; Medeiros, L.J.; Huh, Y.O. Blastic Plasmacytoid Dendritic Cell Neoplasm with Unusual Morphology, MYC Rearrangement and TET2 and DNMT3A Mutations. Br. J. Haematol. 2018, 181, 305.
- Boddu, P.C.; Wang, S.A.; Pemmaraju, N.; Tang, Z.; Hu, S.; Li, S.; Xu, J.; Medeiros, L.J.; Tang, G. 8q24/MYC Rearrangement Is a Recurrent Cytogenetic Abnormality in Blastic Plasmacytoid Dendritic Cell Neoplasms. Leuk. Res. 2018, 66, 73–78.
- Chang, K.-C.; Yu-Yun Lee, J.; Sakamoto, K.; Baba, S.; Takeuchi, K. Blastic Plasmacytoid Dendritic Cell Neoplasm with Immunoblastoid Morphology and MYC Rearrangement and Overexpression. Pathology 2019, 51, 100–102.
- Zachariadis, V.; Schoumans, J.; Barbany, G.; Heyman, M.; Forestier, E.; Johansson, B.; Nordenskjöld, M.; Nordgren, A. Homozygous Deletions of CDKN2A Are Present in All Dic(9;20)(P13·2;Q11·2)-Positive B-Cell Precursor Acute Lymphoblastic Leukaemias and May Be Important for Leukaemic Transformation. Br. J. Haematol. 2012, 159, 488–491.
- Kubota, S.; Tokunaga, K.; Umezu, T.; Yokomizo-Nakano, T.; Sun, Y.; Oshima, M.; Tan, K.T.; Yang, H.; Kanai, A.; Iwanaga, E.; et al. Lineage-Specific RUNX2 Super-Enhancer Activates MYC and Promotes the Development of Blastic Plasmacytoid Dendritic Cell Neoplasm. Nat. Commun. 2019, 10, 1653.
- Hammond, D.W.; Goepel, J.R.; Aitken, M.; Hancock, B.W.; Potter, A.M.; Goyns, M.H. Cytogenetic Analysis of a United Kingdom Series of Non-Hodgkins Lymphomas. Cancer Genet. Cytogenet. 1992, 61, 31–38.
- Jegalian, A.G.; Buxbaum, N.P.; Facchetti, F.; Raffeld, M.; Pittaluga, S.; Wayne, A.S.; Jaffe, E.S. Blastic Plasmacytoid Dendritic Cell Neoplasm in Children: Diagnostic Features and Clinical Implications. Haematologica 2010, 95, 1873–1879.
- Quelen, C.; Lippert, E.; Struski, S.; Demur, C.; Soler, G.; Prade, N.; Delabesse, E.; Broccardo, C.; Dastugue, N.; Mahon, F.-X.; et al. Identification of a Transforming MYB-GATA1 Fusion Gene in Acute Basophilic Leukemia: A New Entity in Male Infants. Blood 2011, 117, 5719–5722.
- Clappier, E.; Cuccuini, W.; Kalota, A.; Crinquette, A.; Cayuela, J.-M.; Dik, W.A.; Langerak, A.W.; Montpellier, B.; Nadel, B.; Walrafen, P.; et al. The C-MYB Locus Is Involved in Chromosomal Translocation and Genomic Duplications in Human T-Cell Acute Leukemia (T-ALL), the Translocation Defining a New T-ALL Subtype in Very Young Children. Blood 2007, 110, 1251–1261.
- Persson, M.; Andrén, Y.; Mark, J.; Horlings, H.M.; Persson, F.; Stenman, G. Recurrent Fusion of MYB and NFIB Transcription Factor Genes in Carcinomas of the Breast and Head and Neck. Proc. Natl. Acad. Sci. USA 2009, 106, 18740–18744.
- Brayer, K.J.; Frerich, C.A.; Kang, H.; Ness, S.A. Recurrent Fusions in MYB and MYBL1 Define a Common, Transcription Factor-Driven Oncogenic Pathway in Salivary Gland Adenoid Cystic Carcinoma. Cancer Discov. 2016, 6, 176–187.
- Zhang, J.; Wu, G.; Miller, C.P.; Tatevossian, R.G.; Dalton, J.D.; Tang, B.; Orisme, W.; Punchihewa, C.; Parker, M.; Qaddoumi, I.; et al. Whole-Genome Sequencing Identifies Genetic Alterations in Pediatric Low-Grade Gliomas. Nat. Genet. 2013, 45, 602–612.
- Sapienza, M.R.; Abate, F.; Melle, F.; Orecchioni, S.; Fuligni, F.; Etebari, M.; Tabanelli, V.; Laginestra, M.A.; Pileri, A.; Motta, G.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm: Genomics Mark Epigenetic Dysregulation as a Primary Therapeutic Target. Haematologica 2019, 104, 729–737.
- Brunetti, L.; Di Battista, V.; Venanzi, A.; Schiavoni, G.; Martelli, M.P.; Ascani, S.; Mecucci, C.; Tiacci, E.; Falini, B. Blastic Plasmacytoid Dendritic Cell Neoplasm and Chronic Myelomonocytic Leukemia: A Shared Clonal Origin. Leukemia 2017, 31, 1238–1240.
- Graubert, T.A.; Shen, D.; Ding, L.; Okeyo-Owuor, T.; Lunn, C.L.; Shao, J.; Krysiak, K.; Harris, C.C.; Koboldt, D.C.; Larson, D.E.; et al. Recurrent Mutations in the U2AF1 Splicing Factor in Myelodysplastic Syndromes. Nat. Genet. 2011, 44, 53–57.
- Ilagan, J.O.; Ramakrishnan, A.; Hayes, B.; Murphy, M.E.; Zebari, A.S.; Bradley, P.; Bradley, R.K. U2AF1 Mutations Alter Splice Site Recognition in Hematological Malignancies. Genome Res. 2015, 25, 14–26.
- Itzykson, R.; Kosmider, O.; Renneville, A.; Morabito, M.; Preudhomme, C.; Berthon, C.; Adès, L.; Fenaux, P.; Platzbecker, U.; Gagey, O.; et al. Clonal Architecture of Chronic Myelomonocytic Leukemias. Blood 2013, 121, 2186–2198.
- Pellagatti, A.; Boultwood, J. Splicing Factor Mutant Myelodysplastic Syndromes: Recent Advances. Adv. Biol. Regul. 2020, 75, 100655.
- Suma, S.; Sakata-Yanagimoto, M.; Nguyen, T.B.; Hattori, K.; Sato, T.; Noguchi, M.; Nannya, Y.; Ogawa, S.; Watanabe, R.; Fujimoto, M.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm Arising from Clonal Hematopoiesis. Int. J. Hematol. 2018, 108, 447–451.
- Alayed, K.; Patel, K.P.; Konoplev, S.; Singh, R.R.; Routbort, M.J.; Reddy, N.; Pemmaraju, N.; Zhang, L.; Shaikh, A.A.; Aladily, T.N.; et al. TET2 Mutations, Myelodysplastic Features, and a Distinct Immunoprofile Characterize Blastic Plasmacytoid Dendritic Cell Neoplasm in the Bone Marrow. Am. J. Hematol. 2013, 88, 1055–1061.
- Togami, K.; Chung, S.S.; Madan, V.; Booth, C.A.G.; Kenyon, C.M.; Cabal-Hierro, L.; Taylor, J.; Kim, S.S.; Griffin, G.K.; Ghandi, M.; et al. Sex-Biased ZRSR2 Mutations in Myeloid Malignancies Impair Plasmacytoid Dendritic Cell Activation and Apoptosis. Cancer Discov. 2022, 12, 522–541.
- Stengel, A.; Baer, C.; Walter, W.; Meggendorfer, M.; Kern, W.; Haferlach, T.; Haferlach, C. Mutational Patterns and Their Correlation to CHIP-Related Mutations and Age in Hematological Malignancies. Blood Adv. 2021, 5, 4426–4434.
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152.
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal Hematopoiesis of Indeterminate Potential and Its Distinction from Myelodysplastic Syndromes. Blood 2015, 126, 9–16.
- Pagano, L.; Valentini, C.G.; Pulsoni, A.; Fisogni, S.; Carluccio, P.; Mannelli, F.; Lunghi, M.; Pica, G.; Onida, F.; Cattaneo, C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasm with Leukemic Presentation: An Italian Multicenter Study. Haematologica 2013, 98, 239–246.
- Laribi, K.; Baugier de Materre, A.; Sobh, M.; Cerroni, L.; Valentini, C.G.; Aoki, T.; Suzuki, R.; Takeuchi, K.; Frankel, A.E.; Cota, C.; et al. Blastic Plasmacytoid Dendritic Cell Neoplasms: Results of an International Survey on 398 Adult Patients. Blood Adv. 2020, 4, 4838–4848.
- Wang, P.; Feng, Y.; Deng, X.; Liu, S.; Qiang, X.; Gou, Y.; Li, J.; Yang, W.; Peng, X.; Zhang, X. Tumor-Forming Plasmacytoid Dendritic Cells in Acute Myelocytic Leukemia: A Report of Three Cases and Literature Review. Int. J. Clin. Exp. Pathol. 2017, 10, 7285–7291.
- Patnaik, M.M.; Lasho, T.L.; Vijayvargiya, P.; Finke, C.M.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Tefferi, A. Prognostic Interaction between ASXL1 and TET2 Mutations in Chronic Myelomonocytic Leukemia. Blood Cancer J. 2016, 6, e385.
- Shih, A.H.; Abdel-Wahab, O.; Patel, J.P.; Levine, R.L. The Role of Mutations in Epigenetic Regulators in Myeloid Malignancies. Nat. Rev. Cancer 2012, 12, 599–612.
- Abdel-Wahab, O.; Levine, R.L. Mutations in Epigenetic Modifiers in the Pathogenesis and Therapy of Acute Myeloid Leukemia. Blood 2013, 121, 3563–3572.
- Tefferi, A. Novel Mutations and Their Functional and Clinical Relevance in Myeloproliferative Neoplasms: JAK2, MPL, TET2, ASXL1, CBL, IDH and IKZF1. Leukemia 2010, 24, 1128–1138.
- Im, A.P.; Sehgal, A.R.; Carroll, M.P.; Smith, B.D.; Tefferi, A.; Johnson, D.E.; Boyiadzis, M. DNMT3A and IDH Mutations in Acute Myeloid Leukemia and Other Myeloid Malignancies: Associations with Prognosis and Potential Treatment Strategies. Leukemia 2014, 28, 1774–1783.
- Summerer, I.; Walter, W.; Meggendorfer, M.; Kern, W.; Haferlach, T.; Haferlach, C.; Stengel, A. Comprehensive Analysis of the Genetic Landscape of 21 Cases with Blastic Plasmacytoid Dendritic Cell Neoplasm by Whole Genome and Whole Transcriptome Sequencing. Leuk. Lymphoma 2021, 62, 2543–2546.
- Yin, C.C.; Pemmaraju, N.; You, M.J.; Li, S.; Xu, J.; Wang, W.; Tang, Z.; Alswailmi, O.; Bhalla, K.N.; Qazilbash, M.H.; et al. Integrated Clinical Genotype-Phenotype Characteristics of Blastic Plasmacytoid Dendritic Cell Neoplasm. Cancers 2021, 13, 5888.
- Fears, S.; Chakrabarti, S.R.; Nucifora, G.; Rowley, J.D. Differential Expression of TCL1 during Pre-B-Cell Acute Lymphoblastic Leukemia Progression. Cancer Genet. Cytogenet. 2002, 135, 110–119.
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. Blood 2017, 129, 424–447.
- Garnache-Ottou, F.; Vidal, C.; Biichlé, S.; Renosi, F.; Poret, E.; Pagadoy, M.; Desmarets, M.; Roggy, A.; Seilles, E.; Soret, L.; et al. How Should We Diagnose and Treat Blastic Plasmacytoid Dendritic Cell Neoplasm Patients? Blood Adv. 2019, 3, 4238–4251.
- Taylor, J.; Haddadin, M.; Upadhyay, V.A.; Grussie, E.; Mehta-Shah, N.; Brunner, A.M.; Louissaint, A.; Lovitch, S.B.; Dogan, A.; Fathi, A.T.; et al. Multicenter Analysis of Outcomes in Blastic Plasmacytoid Dendritic Cell Neoplasm Offers a Pre-Targeted Therapy Benchmark. Blood 2019, 134, 678–687.
- Sumarriva Lezama, L.; Chisholm, K.M.; Carneal, E.; Nagy, A.; Cascio, M.J.; Yan, J.; Chang, C.-C.; Cherry, A.; George, T.I.; Ohgami, R.S. An Analysis of Blastic Plasmacytoid Dendritic Cell Neoplasm with Translocations Involving the MYC Locus Identifies t(6;8)(P21;Q24) as a Recurrent Cytogenetic Abnormality. Histopathology 2018, 73, 767–776.
This entry is offline, you can click here to edit this entry!
