Neuropsychiatric systemic lupus erythematosus (NPSLE) has a broad spectrum of sub-types with diverse severities and prognoses. Ischemic and inflammatory mechanisms, including autoantibodies and cytokine-mediated pathological processes, are key components of the pathogenesis of NPSLE. Additional brain-intrinsic elements (such as the brain barrier and resident microglia) are also important facilitators of NPSLE. An improved understanding of NPSLE may provide further options for managing this disease. The attenuation of neuropsychiatric disease in mouse models demonstrates the potential for novel targeted therapies. Conventional therapeutic algorithms include symptomatic, anti-thrombotic, and immunosuppressive agents that are only supported by observational cohort studies, therefore performing controlled clinical trials to guide further management is essential and urgent. The latest pathogenetic mechanisms of NPSLE are presented, and the progress in its management is discussed.
- neuropsychiatric lupus erythematosus
- pathogenesis
- management
1. Pathogenesis of NPSLE
The exact immunopathogenesis of neuropsychiatric systemic lupus erythematosus (NPSLE) is complex and unclear. Ischemic and autoimmune-mediated neuroinflammatory pathways are now considered two main, and probably complementary, pathogenetic mechanisms leading to NPSLE.
1.1. Ischemic Pathway
Ischemic injury to large- and small- blood vessels, mediated by antiphospholipid (aPL) antibodies, immune complexes, and complement activation leads to focal (e.g., stroke) and diffuse (e.g., cognitive dysfunction) neuropsychiatric events. Among these, aPL antibodies play a predominant role in the intravascular thrombosis [1]. Some studies have reported that SLE patients positive for aPL antibodies are approximately twice as likely to develop NPSLE than aPL-negative patients. aPL antibodies may also increase the risk of subclinical atherosclerosis, leading to a propensity for cerebral ischemia. The central nervous system is more susceptible than most tissues to thrombus formation, which accounts for the increased risk of stroke and transient ischemic attack seen in aPL antibody-positive patients [2]. Apart from thrombosis, aPL antibody positivity has also been correlated with other NPSLE manifestations, such as seizures, chorea, cognitive dysfunction, and myelopathy [3][4][5], especially psychosis [6][7][8]. Recent evidence suggests that aPL antibodies are also linked to direct neuronal damage by inducing oxidative stress and damage to neuronal cell membranes via the β2-glycoprotein. In an in vitro study, aPL antibodies bound to neurons and other CNS cells, and the intracerebroventricular injection of aPL induced a hyperactive behavior in animal models [9], thereby supporting a direct effect of these antibodies on the brain.
1.2. Neuroinflammatory Pathway
Autoimmune-mediated neuroinflammatory pathways with complement activation, enhanced the permeability of the blood–brain barrier (BBB), the intrathecal migration of neuronal autoantibodies, and the local production of pro-inflammatory cytokines, and other inflammatory mediators are associated with mostly diffuse neuropsychiatric manifestations, such as psychosis, mood disorders, and cognitive dysfunction [10][11][12][13].
2. Current Management of NPSLE
The management of NPSLE can be challenging, because of the complexity of its pathogenesis, difficulty in its accurate diagnosis, and a lack of clinical trials in NPSLE. Current treatment options for NPSLE are usually derived from observational studies and refer to the experience of treatment of other SLE subtypes, such as lupus nephritis and similar neuropsychiatric disorders [10][14].
First, symptomatic therapy is necessary: anti-epileptics for seizures, and anxiolytics, antidepressants, mood-stabilizers, or antipsychotics should be administered as appropriate. Neurotrophic and neuroleptic agents were generally adopted in case with peripheral nervous system involvement [15]. The treatment of the underlying SLE process should be undertaken based on whether the pathogenesis is primarily related to an inflammatory or ischemic disease pathway (Figure 1).
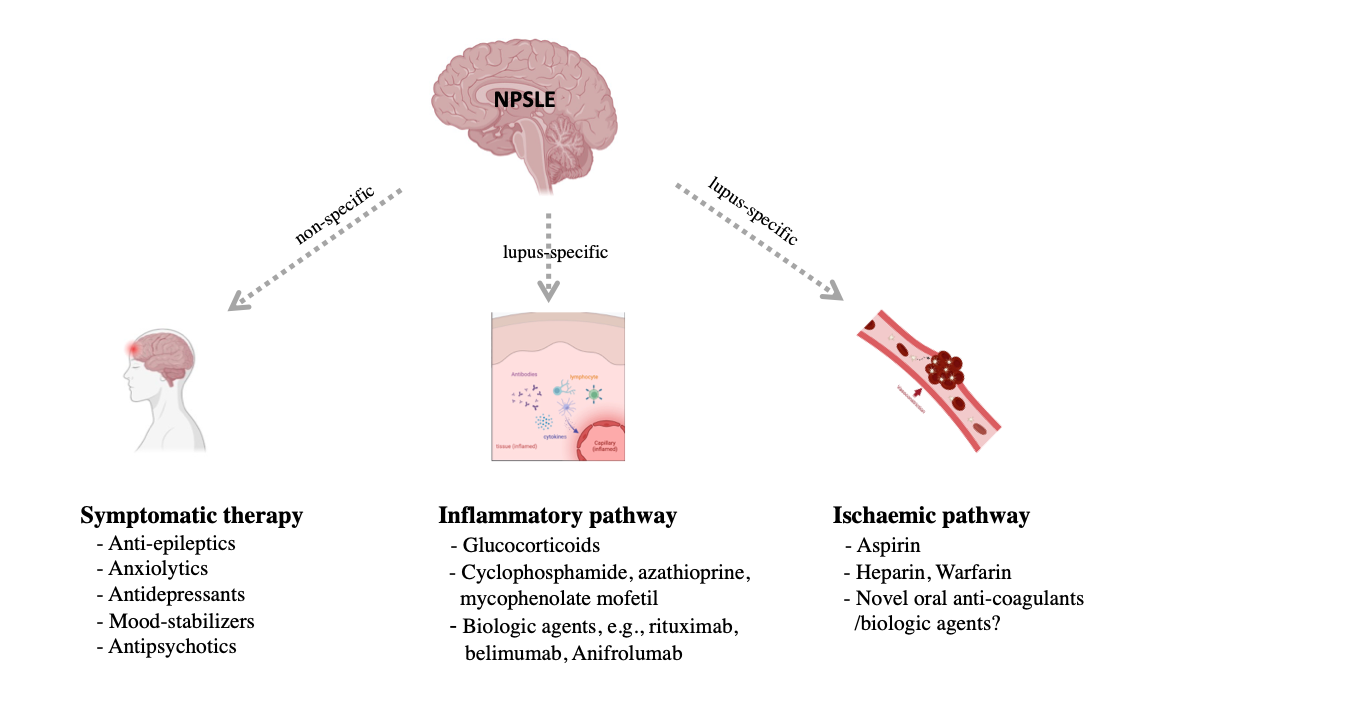
Figure 1. Management for patients with neuropsychiatric systemic lupus erythematosus (NPSLE). Created with BioRender.com.
2.1. Inflammatory pathway therapies
Glucocorticoids have been a cornerstone in the treatment of various manifestations of NPSLE, especially in those associated with an immune-inflammatory pathogenesis [16][17]. High-dose glucocorticoids, alone or in combination with cyclophosphamide, azathioprine, and mycophenolate mofetil, are reported to be effective, but their use is mainly based on patient’s clinical experience of disease severity and their clinician’s preference. Given the evidence linking glucocorticoids use to cumulative organ damage in SLE [18] and its associated psychiatric symptoms [19][20], alternative therapeutic strategies are essential.
2.2. Ischemic Pathway Therapies
Cerebral ischemia attributed to NPSLE, such as transient ischemic attacks and stroke attributed to NPSLE events and is thought to be correlated with aPL antibodies. Thus, the primary prevention of cerebral ischemia in NPSLE is linked to a reduction in prothrombotic risk.
3. Promising Targeted Therapies
Due to the lack of understanding of the exact pathogenic mechanisms behind this condition as well as its diverse neuropsychiatric manifestations, we have limited experience in targeted therapies for patients with NPSLE. The attenuation of neuropsychiatric diseases in related animal models demonstrates the potential for targeted therapies, which are based on a current understanding of the pathogenesis of NPSLE (Table 1).
Table 1. Promising targeted therapies in NPSLE.
|
Promising Targeted Therapies |
Underlying Mechanisms and Clinical Findings |
Experimental Arrangement |
Potential Drugs |
|
Complement inhibitors |
Complement signaling promotes the loss of BBB integrity. |
Human brain autopsies |
Eculizumab |
|
BBB-targeted therapy |
BBB disruption is essential in the neuronal damage process. |
Human and mouse cells; |
GW0742, a peroxisome proliferator-activated receptor β/δ agonist; KD025, a rho kinase inhibitor. |
|
MMPs inhibitors |
There is an association between CSF/serum levels of MMP-9, psychiatric NPSLE, and markers for neuronal/astrocytic damage. MMP-9 may contribute to the pathogenesis of psychiatric NPSLE by stimulating T-cell migration |
- |
- |
|
IFN-α/β receptor antagonists |
IFN receptor inhibition decreased microglia-related synaptic loss and attenuated anxiety-like behavior and cognitive deficits in animal models. |
564Igi lupus-prone mice |
Anifrolumab |
|
BTK inhibitors |
Use of BI-BTK-1 (an inhibitor of BTK) in MRL/lpr mice, decreased the infiltration of macrophages, T cells, and B cells in the choroid plexus, and improved cognitive function. |
MRL/lpr mice |
Ibrutinib; Evobrutinib |
|
S1P receptor modulator |
S1P receptor modulators decreased proinflammatory cytokine secretion by microglia and significantly improved spatial memory and depression-like behavior. |
MRL/lpr mice |
Fingolimod |
|
ACE inhibitors |
ACE inhibitors treatment suppressed microglial activation and promoted cognitive status. |
BALB/c mice |
Captopril; Perindopril |
|
CSF1R inhibitors |
CSF1R is essential in both macrophage and microglia function. |
MRL/lpr mice |
GW2580, a small CSF-1R kinase inhibitor; depletion of microglia |
|
Nogo-a/NgR1 antagonists |
Nogo-a/NgR1 in the CSF is significantly increased in NPSLE. |
MRL/lpr mice |
Nogo-66 |
|
JAK inhibitors |
JAK inhibitors penetrate the BBB and reduce the production of several cytokines, including type I IFNs. |
- |
Tofacitinib |
This entry is adapted from the peer-reviewed paper 10.3390/jcm11174955
References
- Ho, R.C.; Thiaghu, C.; Ong, H.; Lu, Y.; Ho, C.S.; Tam, W.W.; Zhang, M.W. A meta-analysis of serum and cerebrospinal fluid autoantibodies in neuropsychiatric systemic lupus erythematosus. Autoimmun. Rev. 2016, 15, 124–138.
- Ben Salem, C. The pathogenesis of the antiphospholipid syndrome. N. Engl. J. Med. 2013, 368, 2334.
- Mikdashi, J.; Handwerger, B. Predictors of neuropsychiatric damage in systemic lupus erythematosus: Data from the Maryland lupus cohort. Rheumatology 2004, 43, 1555–1560.
- Andrade, R.M.; Alarcón, G.S.; González, L.A.; Fernández, M.; Apte, M.; Vilá, L.M.; McGwin, G., Jr.; Reveille, J.D. Seizures in patients with systemic lupus erythematosus: Data from LUMINA, a multiethnic cohort (LUMINA LIV). Ann. Rheum. Dis. 2008, 67, 829–834.
- Tomietto, P.; Annese, V.; D’Agostini, S.; Venturini, P.; La Torre, G.; De Vita, S.; Ferraccioli, G.F. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 2007, 57, 1461–1472.
- Shoenfeld, Y.; Nahum, A.; Korczyn, A.D.; Dano, M.; Rabinowitz, R.; Beilin, O.; Pick, C.G.; Leider-Trejo, L.; Kalashnikova, L.; Blank, M.; et al. Neuronal-binding antibodies from patients with antiphospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus 2003, 12, 436–442.
- Caronti, B.; Calderaro, C.; Alessandri, C.; Conti, F.; Tinghino, R.; Pini, C.; Palladini, G.; Valesini, G. Serum anti-beta2-glycoprotein I antibodies from patients with antiphospholipid antibody syndrome bind central nervous system cells. J. Autoimmun. 1998, 11, 425–429.
- Chapman, J.; Cohen-Armon, M.; Shoenfeld, Y.; Korczyn, A.D. Antiphospholipid antibodies permeabilize and depolarize brain synaptoneurosomes. Lupus 1999, 8, 127–133.
- Katzav, A.; Ben-Ziv, T.; Blank, M.; Pick, C.G.; Shoenfeld, Y.; Chapman, J. Antibody-specific behavioral effects: Intracerebroventricular injection of antiphospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J. Neuroimmunol. 2014, 272, 10–15.
- Schwartz, N.; Stock, A.D.; Putterman, C. Neuropsychiatric lupus: New mechanistic insights and future treatment directions. Nat. Rev. Rheumatol. 2019, 15, 137–152.
- Hanly, J.G.; Kozora, E.; Beyea, S.D.; Birnbaum, J. Review: Nervous System Disease in Systemic Lupus Erythematosus: Current Status and Future Directions. Arthritis Rheumatol. 2019, 71, 33–42.
- Kowal, C.; DeGiorgio, L.A.; Nakaoka, T.; Hetherington, H.; Huerta, P.T.; Diamond, B.; Volpe, B.T. Cognition and immunity; antibody impairs memory. Immunity 2004, 21, 179–188.
- Bravo-Zehnder, M.; Toledo, E.M.; Segovia-Miranda, F.; Serrano, F.G.; Benito, M.J.; Metz, C.; Retamal, C.; Álvarez, A.; Massardo, L.; Inestrosa, N.C.; et al. Anti-ribosomal P protein autoantibodies from patients with neuropsychiatric lupus impair memory in mice. Arthritis Rheumatol. 2015, 67, 204–214.
- Li, M.; Zhao, Y.; Zhang, Z.; Huang, C.; Liu, Y.; Gu, J.; Zhang, X.; Xu, H.; Li, X.; Wu, L.; et al. 2020 Chinese guidelines for the diagnosis and treatment of systemic lupus erythematosus. Rheumatol. Immunol. Res. 2020, 1, 5–23.
- Bortoluzzi, A.; Piga, M.; Silvagni, E.; Chessa, E.; Mathieu, A.; Govoni, M. Peripheral nervous system involvement in systemic lupus erythematosus: A retrospective study on prevalence, associated factors and outcome. Lupus 2019, 28, 465–474.
- Barile-Fabris, L.; Ariza-Andraca, R.; Olguín-Ortega, L.; Jara, L.J.; Fraga-Mouret, A.; Miranda-Limón, J.M.; Fuentes de la Mata, J.; Clark, P.; Vargas, F.; Alocer-Varela, J. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann. Rheum. Dis. 2005, 64, 620–625.
- Denburg, S.D.; Carbotte, R.M.; Denburg, J.A. Corticosteroids and neuropsychological functioning in patients with systemic lupus erythematosus. Arthritis Rheum. 1994, 37, 1311–1320.
- Ruiz-Arruza, I.; Lozano, J.; Cabezas-Rodriguez, I.; Medina, J.A.; Ugarte, A.; Erdozain, J.G.; Ruiz-Irastorza, G. Restrictive Use of Oral Glucocorticoids in Systemic Lupus Erythematosus and Prevention of Damage Without Worsening Long-Term Disease Control: An Observational Study. Arthritis Care Res. 2018, 70, 582–591.
- Bolanos, S.H.; Khan, D.A.; Hanczyc, M.; Bauer, M.S.; Dhanani, N.; Brown, E.S. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann. Allergy Asthma Immunol. 2004, 92, 500–505.
- Lewis, D.A.; Smith, R.E. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J. Affect. Disord. 1983, 5, 319–332.
