Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemical Research Methods
Lipases are efficient enzymes with promising applications in the nutraceutical and food industry, as they can offer high yields, pure products under achievable reaction conditions, and are an environmentally friendly option.
- lipases
- food
- nutraceutical industry
1. Lipases as Biocatalysts in the Food and Nutraceutical Industry
Lipases are widely used in the food industry [9,10]. Lipases (triacylglycerol hydrolases EC 3.1. 1.3) play a crucial role in numerous industrial food processes [11,12] because they participate in reactions that improve product quality and provide greater stability, solubility, durability, and better organoleptic characteristics [10,13,14].
1.1. Lipase Characteristics
These enzymes can hydrolyze triglycerides to obtain free fatty acids, monoacylglycerols (MAGs), diacylglycerols (DAGs), and glycerol; on the other hand, they can synthesize new products in organic media by esterification, transesterification, and aminolysis mechanisms (Figure 1) [15,16]. Lipases have a highly conserved catalytic triad comprising serine as a nucleophile, an aspartate/glutamate as an acidic residue, and histidine. In their active conformation, lipases present in their active center a group of hydrophobic residues arranged around the catalytic serine that constitute an electrophilic region known as an oxyanion cavity. Lipases are also characterized by the presence of disulfide bridges that give them stability and are critical for their catalytic activity [16]. Some lipases also have a structural feature covering the active site, called the “lid,” that opens at hydrophobic/hydrophilic interphases. Ancient classifications denoted esterases as lipolytic enzymes lacking a lid. However, because some lipases, such as Candida antarctica lipase B (CALB), lack the lid, an alternative classification has been proposed [17].
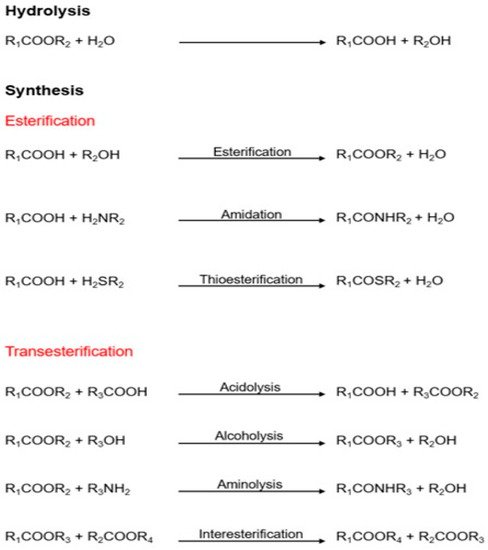
Figure 1. Different reactions are catalyzed by lipases [15].
Lipases are characterized by maintaining their activity and high production in nonaqueous media [18], high production, and stability at pH ranges and do not require cofactors. According to their substrate specificity, lipases can be chemoselective, regioselective, or stereoselective. The first lipase type can selectively catalyze a reaction. The second type catalyzes a reaction specifically with one of the triglyceride positions (sn-1,3 regioselective, sn-2 regioselective, or nonregioselective). Additionally, the third type catalyzes reactions selecting only one of the stereoisomers from a mixture of enantiomers [10,16].
1.2. Sources and Tools to Improve Lipase-Catalyzed Reactions
Lipases are ubiquitous enzymes produced by various organisms, including microorganisms, plants, and animals [12,19,20,21,22,23,24]. Because of the increased commercial interest in these proteins in the food and nutraceutical industry, the use of recombinant production technology is critical.
The productivity of lipase production bioprocesses has been increasing, reducing the cost of enzymes by using cell factories for the heterologous production of lipases. Between them, Komogataella phaffi (P. pastoris) is one of the most common cell factories used [25].
Lipases have been improved using natural evolution techniques, protein engineering, bioinformatics design, directed evolution, saturation mutagenesis, site-directed mutagenesis, and DNA shuffling [26]. However, in the food industry, the native form is often preferred (Figure 2).
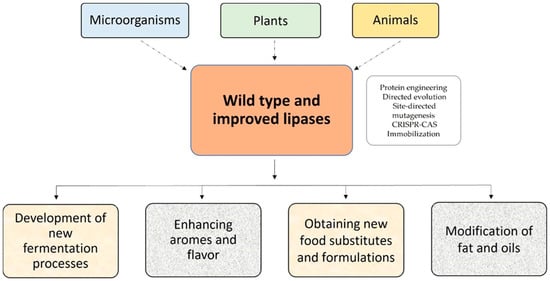
Figure 2. Sources and use of lipases in the food industry.
Table 1 shows some microbial lipases that are commercially available and immobilized on different supports to enhance their efficiency and reuse [27,28,29,30]. Most commercially important lipase-producing yeasts belong to the class of ascomycetes, such as Candida sp. and Rhizopus sp. Novozymes® (Bagsværd, Denmark), DuPont® (Wilmington, DE, USA), Roche® (Basel, Switzerland), and Amano (Yokohama, Japan) are the main companies that produce and commercialize lipases [31].
Table 1. Sources of lipases with applications in food and nutraceutical industry.
| Source/Commercial Name | Type | Application/Products | Reference |
|---|---|---|---|
| Candida antarctica lipase B (CALB)/Novozym 435/Lipozyme 435 | Recombinant | Flavor esters | [32] |
| Candida rugosa | Wild type | Glycerides, production flavor compounds | [33,34] |
| Termomyces lanuginosus/Lipozyme TL IM | Engineered | Food formulation, Interesterification of fats and oils | [35,36] |
| Aspergillus sp. | Wild type | Flavor and fragance | [37] |
| Aspergillus oryzae | Wild type | Interesterification of fats and oils | [36] |
| Geotrichum candidum | Wild type | Oil with increased unsaturation | [36] |
| Rhizomucor miehei/Lipozyme RM IM | Recombinant | Enhancing fruit fragrance | [38] |
| Modification of the amount and composition of volatile components in bovine milk | [39] | ||
| Ras Cheese Flavor Concentrate (RCFC) | [40] | ||
| Rhizopus oryzae | Wild type | Human Milk Fat Substitutes | [41] |
| Lactococcus chungangensis | Wild type | Flavoring in milk, cream cheese, yogurt and butter. | [42] |
| Lactobacillus plantarum | Wild type | Fermented food and cheese | [43,44] |
| Staphylococcus epidermidis | Wild type | Flavor-compound production | [45] |
| Ophiostoma piceae | Wild type | Flavor-compound production | [46] |
| Meyerozyma guilliermondii | Wild type | Feed industry | [47] |
Other important bottlenecks of the free enzymes in general and lipases are the low operational stability in synthesis reactions using solvents and substrates such as alcohols and organic acids, the high cost of the enzymes, and the need to reuse the biocatalyst minimizing product separation.
Different approaches are being applied (Figure 2) to solve these drawbacks. The use of enzyme immobilization methods normally increases biocatalyst stability, specificity and selectivity, allows the reutilization of the enzyme, and minimizes downstream processes, and has been reflected in the number of articles and patents published in this field [48].
Advances in the study of lipases seek to develop more efficient processes and, for this purpose, their stability under certain temperatures, solvents, and pH conditions, among others. The development of a specific reaction medium to increase the activity, stability, and productivity of biocatalysts has been a recurring topic of research over the last three decades. The remarkable properties and useful applications of enzymes, particularly lipases, have inspired various strategies to improve their performance in near-anhydrous media. Therefore, medium engineering can be used to modulate the activity and selectivity of lipase-catalyzed reactions [49].
Ionic liquids (ILs) are molten salts that originate from the association of organic cations and organic/inorganic anions. The use of ILs as solvents in biocatalysis processes has recently received increased attention, and substantial progress has been made, particularly in lipase-catalyzed reactions. ILs have the advantages of low volatility, low inflammability, and a low melting point [50]. Deep eutectic solvents [51] are eutectic mixtures of salts and hydrogen bond donors with sufficiently low melting points to act as solvents. DESs were demonstrated to be a viable alternative to traditional organic solvents and ILs in many biocatalytic processes, particularly for lipases. DESs have additional advantages over ILs in simple preparation and lower costs because of their renewable and readily available raw materials [52].
2. Established Applications of Lipases in the Food and Nutraceutical Industry
Lipases in the food industry and nutraceutical production can be used in aqueous extracts and purified, immobilized, or whole cells to exploit the available raw material and increase their economic and nutritional value. These enzymes can be used to modify fats and oils and synthesize structured lipids or antioxidants with increased antioxidant power or modified lipophilicity, flavors, and aromas [53,54].
2.1. Fats and Oils
Patent searches suggest that lipase has an impressive number of applications in the modification of fats and oils and enhancement of flavor in food products—e.g., cheese, butter, milk, and chocolate [55]. Some applications of lipases in dairy products and the synthesis of structured lipids are described in the following sections.
2.1.1. Dairy Products
In the dairy sector, lipases are used to provide desirable aromatic characteristics to cheddar, provolone, and Romano cheeses conferred by these free short-chain fatty acids generated in the hydrolysis of fats [40,56]. Recent advances have allowed the biosynthesis of short-chain ethyl esters with fruity notes in whole milk by coupling ethanolic fermentation with transesterification using the commercial lipase Palatase. For fermentation, the following microorganisms were used: Kluyveromyces marxianus, Lactobacillus fermentum, and L. Paracasei. Many esters were obtained in ethanolic fermentation using K. marxianus yeast and lipase. This method of milk fermentation and lipase addition represents a new alternative for flavoring milk [57].
2.1.2. Structured Lipids
In recent years, structured lipids have become a topic of great importance in the food and nutraceutical industry because technological advances allow a generation of products of better quality and that better meet consumer demands. Within this innovation in food processes, structured lipids (SLs) have been generated [58,59].
Structured lipids are fats and oils whose fatty acid composition has been modified for nutritional purposes to achieve greater bioavailability because they are not naturally occurring. In several cases, lipids have certain limitations of use in their original state because of the specific composition of their fatty acids [60].
In other cases, even when they are available as raw materials, they cannot always meet nutritional demand, e.g., restrictions on the daily intake of saturated fatty acids and trans fatty acids have been increased because they are related to cardiovascular diseases [61,62]. Another clear example would be access to cocoa butter; its availability may be limited by external factors such as climate change, fluctuating prices, and availability [63,64]. Therefore, the search for alternatives to address these major issues is justified.
In principle, deciding which type of fatty acids to use and in which position of the molecule to restructure is possible by obtaining structured lipids [58]. For this procedure, the use of stereospecific enzymes allows new lipids with a stable structure to be obtained. Lipases can hydrolyze a triglyceride in an aqueous medium, but they also catalyze the binding of a fatty acid to a glycerol molecule in an anhydrous reaction medium [65]. Recently, the use of immobilized biocatalysts has minimized the production costs of structured lipids through reusing them in successive batches [58].
Human milk fat substitute (HMFS) is synthesized by enzymatic interesterification of vegetable oils, animal fats, or oil mixtures, commonly using an immobilized regioselective lipase in either solvent or solvent-free media [70,73].
A recently reported lipase/acyltransferase from C. parapsilosis was used as a biocatalyst to synthesize HMFS by interesterification of ethyl oleate with tripalmitin in solvent-free media representing a new alternative to commercial immobilized lipases [70]. Because human milk is one of the most complex mixtures of natural lipids, studies using this approach will continue to advance steadily.
2.2. Vitamin Esters
Food contains components known as bioactive compounds that, when consumed, provide energy to the body, promote good health and minimize the risk of disease. The bioactive compounds that are extracted from the original food and maintain their beneficial properties for health are called nutraceuticals [2]. For consumption and consumer acceptance, the functionality of bioactive compounds, safety, and nontoxicity must be guaranteed beforehand [74]. Highlighting a representative example, antioxidants play a crucial role in the food industry because, during food processing, the matrices used mostly incorporate lipids as emulsifiers or additives, making lipid oxidation a challenge to consider [75,76,77,78]. Lipid oxidation involves the attack of molecular oxygen on unsaturated fat molecules, which can generate undesirable volatile flavoring compounds that contribute to rancidity [79]. Even when quality controls are followed during food product preparation and packaging, the rate of lipid oxidation is influenced by several endogenous and exogenous parameters, including oxygen, light metals, and polyunsaturated lipids, primarily because the latter are prone to oxidation [80,81]. Antioxidants are used to mitigate this effect, meaning molecules that reduce, neutralize, or deplete molecular oxygen, remove pro-oxidative metal ions, and scavenge reactive oxygen species (ROS), hydrogen peroxide or superoxide anion radicals [82,83,84,85].
Antioxidants occur naturally, and the best known are ascorbic acid (vitamin C), tocopherols (vitamin E), carotenoids, and thiols [86]. During their absorption in the body, they complement the defense action as they help to slow down the use of endogenous antioxidants and improve the body’s ability to avoid oxidative stress [82,87,88,89,90]. The lack of action of endogenous antioxidants, either by diminution or stress, is related to the modification of lipid membrane components [91], resulting in neurodegenerative, cardiovascular, inflammatory diseases, diabetes, male infertility, and cancers of the breast, lung, liver, colon, prostate, ovary and brain [87,92,93,94,95,96,97]. The excessive presence of reactive oxygen species promotes the expression of oncogenic genes [93]. Antioxidants, as nutraceuticals, play a key role in the nutritional base because of their close relationship with biological processes; thus, skin benefits are also attributed to them for delaying aging [98,99,100]. Although the concept of nutraceuticals is not new, the trend to use antioxidants with biochemical properties of high stability and biocompatibility as a complementary ingredient has become interesting [84,101,102].
2.3. Bakery Products
New requirements in bakery products make the development of new formulations that conform to what would be green or less harmful labels. In bakery products, lipases have been successfully applied to improve dough processing, strength, volume, structure, and softness, decrease stickiness, and increase the quality and shelf life [138,139].
With a focus on the intermediate product of bread, dough plays an indispensable role in becoming the final product because it is a semisolid foam that is converted into a solid cellular sponge upon baking so that the mixture of the lipid fraction of wheat, eggs, or baker’s fat exerts major roles in gas incorporation and its stabilization, which are necessary to achieve a fluffy product [140].
Although wheat flour contains low levels of lipids, they affect the quality of fresh bread because they are related to storage duration. Briefly, the studies are directed toward knowledge of the relationship of the flours or their reformulation by adding lipids from other sources and their effect on quality.
Recently, lipases have been successfully applied to investigate how endogenous or exogenous lipids affect bread making. Lipases hydrolyze galactolipids, and their presence in the dough improves bread volume. The flour was defatted and subsequently reconstituted by adding different fractions of these lipids to determine the relationship of endogenous lipids in wheat flour and their impact on bread volume. The hydrolysis of endogenous lipids and their enzymatically released products are responsible for the positive effects on bread [138,141].
To understand the role of endogenous wheat lipids on the evolution of bread crumb firmness during storage, three lipases—Lipopan F, Lecitase Ultra, and Lipolase—were evaluated, and sodium lactylate stearoyl surfactant (SSL) was used as a surfactant. By forming amylose-lipid (AM-L) inclusion complexes, the surfactants retarded bread crumb firming. Some endogenous wheat lipids have surfactant-like structures, so the use of enzymes in bread making would increase the level of free fatty acids that allow the formation of amylose-lipid complexes. The evaluation of three enzymes showed that lipases and SSL similarly affected the texture of breadcrumbs during storage. However, after seven days of storage, the sample containing Lipolase significantly reduced amylopectin retrogradation, evidencing the importance of the formation of amylose-lipid inclusion complexes. Therefore, lipases have been proposed as alternatives to surfactants because they produce molecules in situ that possess hydrophilic and hydrophobic structures like those of surfactants [141,142].
2.4. Flavors and Fragances
In the world market, a high demand exists for fragrance and flavor esters for different industries, including food, cosmetics, and pharma, as ingredients of many products (food, beverages, candies, jellies, jams, wines, dairy products, perfumes, body lotions, shampoos, and other toiletries) [143,144]. The flavor and fragrance market was valued at $28 billion in 2019 and is expected to expand at a compound annual growth rate (CAGR) of 4.7% to $35 billion from 2021 to 2027 [145]. Another characteristic is that many of these products are chiral [146]. This potential chiral product can be consulted in the database [147].
Many of these products are obtained after extraction from their natural sources (plants, fruits, and flowers). However, the low concentration of these products in their natural sources, climatic dependence of the source, and low yield and high production cost of the extraction and purification phases make it challenging to assume an increased world demand [143].
A wide range of flavors and fragrances can be obtained by chemical synthesis, solving the of raw material producing the same products at a lower cost. However, these products have not been labeled as natural according to European legislation (EC 1334/2008), and obtaining pure chiral compounds is challenging. In this context, the substitution of a chemical using biotechnology (microbial biosynthesis or applied biocatalysis) is being widely explored because the products can be labeled as natural if the employed reactants are labeled as natural. The resolution of chiral compounds is generally higher with no problems in selectivity, reaching higher yields and with an easier downstream due to the absence of undesirable side reactions. However, the operational conditions (P, T) are softer than those of the chemical approach. A marketplace of bioflavors is actually 100–500 $/kg, and more than 100 flavor products are commercialized [148]. In 2019, the global biotech flavor market was close to 0.5 billion US$, approximately 1.5% of the estimated global market in the same year and is expected to grow at a compound annual growth rate (CAGR) of 9.3% from 2020 to 2027. Similarly, biotech vanillin represents ca. 3% of the total vanillin market, and it is speculated to increase at a CAGR exceeding 13% by 2023 [149].
Thus, the significant demand for these esters has boosted the need for greener production routes and food safety aspects for human consumption, making enzymatic synthesis a favorable alternative to chemical catalysts [150,151]. Approximately 4000 enzymes are known, and close to 200 have been mainly commercialized for stereoselective organic synthesis and the biotechnological production of flavor compounds [148]. Between them, lipases are the most applied enzyme family to produce flavor and fragrances. Although their natural biocatalysis is the hydrolysis of lipids to produce free fatty acids, glycerol, or other alcohols, they also work in reactions of esterification and trans- and interesterification and the transfer of acyl groups from esters to other nucleophiles (e.g., amines and thiols) [143,152].
This entry is adapted from the peer-reviewed paper 10.3390/catal12090960
This entry is offline, you can click here to edit this entry!
