The last few years have increasingly emphasized the need to develop, through nanoscale technologies, new active antiviral products useful for infection prevention and control. At the same time, advanced computational approaches have found themselves fundamental in the repurposing of active therapeutics or for reducing the very long developing phases of new drugs discovery, which represents a real limitation, especially in the case of pandemics.
- SARS-CoV-2
- nanosystems
- antiviral activity
1. Introduction
The novel coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), emerged from Wuhan, China in November 2019, is primarily transmitted from person to person via respiratory droplets and aerosols produced when talking, coughing, and sneezing[1], from both symptomatic and asymptomatic people[2][3]. Most patients affected by COVID-19 (Coronavirus disease 2019) develop only mild to moderate symptoms (fever and dry cough). Unfortunately, patients can develop severe illness (dyspnea, respiratory rate ≥ 30 breaths per minute, blood oxygen saturation ≤ 93%, lung infiltrates > 50%), requiring oxygen support, and also become critically ill with respiratory failure, acute respiratory distress syndrome, sepsis, thromboembolism, and multiorgan failure[4][5]. It became evident early on that severe COVID-19 is characterized by uncontrolled systemic hyperinflammation (the so-called cytokine storm), a condition triggered by the release of a big amount of pro-inflammatory cytokines[6].
SARS-CoV-2 shows a continuous evolution due to changes in its genetic code, and multiple variants of this virus have been discovered in the world during this pandemic. A group of variants with similar genetic changes may be designated by public health organizations as Variant of Concern (VOC), Variant of Interest (VOI) and Variant Being Monitored (VBM), if they share characteristics requiring public health action. Many of these variants are mutations concerning the viral Spike protein and therefore, due to the role of this protein in host cell penetration, are the main target of surveillance. Up to today, SARS-CoV-2 still remains a respiratory pathogen with unpredictable viral evolution, aggravated by widespread circulation, intense transmission in humans and appearance of multiple SARS-CoV-2 VOCs, characterized by increased transmissibility and/or virulence, or reduced effectiveness of countermeasures.
Nanoscale technologies are attracting ever-increasing interest from basic and industrial research for their potential employment in diseases due to several viruses[7], and for the development of new effective therapeutic treatments as well as diagnostic tools, innovative materials, and drugs useful for infection prevention and control[8][9][10].
Strategies involving nanodrugs for the treatment and prevention of viral diseases, including COVID-19, are based on the mechanisms that are described below:
Trapping effects: the first step in a viral invasion is its attachment to the host cell membrane. Therefore, it is highly considered when designing anti-infection nanomaterials, including those based on cell membrane properties; in fact, cell-mimicking nanoparticles (NPs), displaying a pathogen’s cognate receptor on their surface, represent an emerging class of therapeutics that are potentially effective against SARS-CoV-2 and other viruses[11].
Inhibition of viral entry: the inhibitory effect of nanodrugs on cell entry by viruses and related mechanisms has been studied extensively. Viral attachment and entry usually require interaction between viral surface protein(s) and receptor(s) on host cell membranes; thus, nanomaterials that interfere with such interactions are promising antiviral agents which can act at a relatively accessible extracellular level, and thus prevent the infection state at an early stage[12]. Some nanodrugs interrupt virus–cell interactions by blocking viral surface proteins and cell membrane receptors[13][14][15]. In particular, SARS-CoV-2 uses the viral Spike protein and the host cell angiotensin-converting enzyme II (ACE2) protein, as the main method of cell penetration, through a “Lock and Key” mechanism that allows the virus access to cells having the corresponding lock. These sites can be found throughout the body, but especially in the lungs, heart, and arteries. This process emphasizes the need to find a treatment that could potentially inhibit this (and similar) “Lock and Key” process(es) altogether.Inhibition of viral replication: nanodrugs can inhibit viral replication by interacting with the viral protein/genome or inducing a suppressive environment for intracellular viral replication.
Viral inactivation effects: nanodrugs, such as metal- and carbon-based nanomaterials, take contact directly with viruses and induce viral inactivation by different mechanisms depending on the nanomaterial and virus. The destruction of the viral envelope or capsid (for example by photocatalytic oxidation) is a common mechanism by which nanomaterials induce the inactivation of viruses.
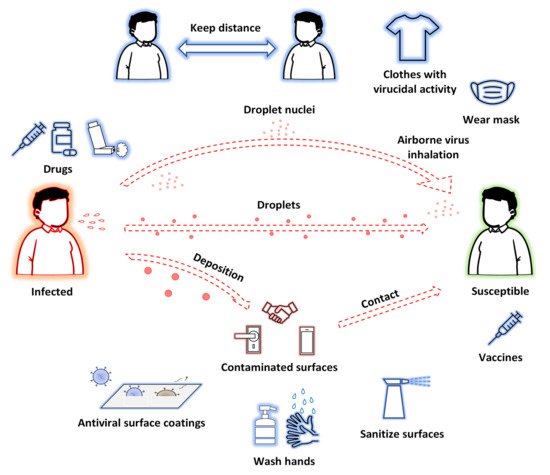
Transmission methods of SARS-CoV-2 and possible approaches to prevent and treat COVID-19 are shown in Figure 1.
.
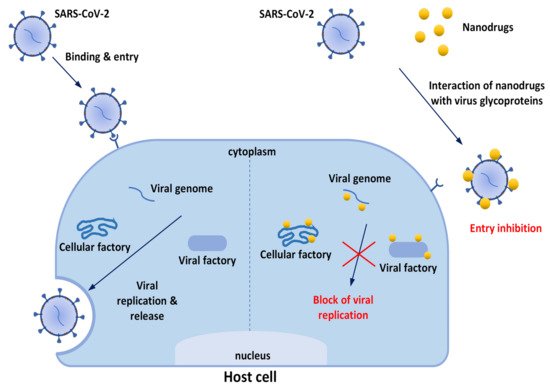
The first issue to be underlined to explain nanotechnologies’ great potential for improving prevention and treatment of viral diseases is that nanomaterials[11] possess unique properties—particularly their small size (1–100 nm), but also their high surface-to-volume ratios and modifiable surfaces— which are beneficial for contact with viruses and contribute to multiple antiviral effects. Nanomaterials have been reported to suppress cell entry and viral replication; moreover, their numerous surface binding sites facilitate interactions with target molecules, consequently trapping and inactivating viruses. So, it is evident that modern nanotechnologies do not solely aim to discover new antiviral therapeutics (or to repurpose known products). Nanocomposites can be grouped with different antiviral agents in order to obtain a synergistic effect. Furthermore, nanomaterials may be used to produce innovative drug delivery systems (DDSs) to improve the pharmacokinetic properties of loaded drugs. If drugs are sensible to external stimuli, they may be projected as endowed with auxiliary functions useful for inactivating viruses or mimicking host cells, or they could be functionalized with specific ligands that are able to bind molecular components of the target, and so on (Figure 2). Finally, there is a strong effort toward the development of nanoproducts to be used for nasal/inhalation therapy and taking advantage of antiviral or anti-inflammatory activity of their ingredients[16][17].
The discovery and development of antiviral nanodrugs take enormous advantages from the employment of high-performance computing-based tools[18][19][20][21]. Advanced computational techniques are fundamental in discovering or repurposing, in a rapid and therefore constantly updated way, active therapeutics obtained via chemical synthesis but also derived from natural (particularly vegetable) matrices. These techniques, indispensable for medicine screening to accelerate the development of specific drugs against SARS-CoV-2, are based on the knowledge of the structure of the possible targets (at the level of the virus and of the host cell), to which the potential drug can competitively bind. In particular, molecular docking calculations are based on receptor active site regions to search for whether ligands interact with the target structure and the optimal binding mode between them; molecular dynamics simulation is a method that simulates experimental conditions and can display the microscopic evolution of the system at an atomic level[19][20].
.
A particular issue to be underlined is that to defeat COVID-19 (although this can be generalized to other transmissible viral and bacterial infectious diseases), in addition to therapeutic treatments, control measures are essential. The recent outbreak of COVID-19 has demonstrated that the adoption of passive measures helps minimize the impact of current and future infection outbreaks, so that innovative nanomaterials may have a main role in the development of virus spread control measures which would be efficient against SARS-CoV-2. For example, research on antiviral textiles has received considerable attention, since these can effectively inhibit the spread of viruses or the formation of biofilms on their surface, reducing the risk of infection/re-infection[22][23][24][25]. When COVID-19 patients cough or sneeze, tiny virus-containing droplets are emitted and can contaminate surrounding surfaces, contributing to virus spread. This has increased researchers’ interest in developing not only new hand and surface disinfectants, but also self-decontaminating surface materials[26]. Herein we have reviewed the most recent and innovative nanomaterials proven to be effective against SARS-CoV-2, as well as possibly against other viruses, and useful to develop passive control virus spread measures [from masks and personal protective equipments (PPE) to disinfectants and surface coatings].
2. Nanomaterials for viral spread control
2.1. Metal-Based Nanomaterials
Several viruses, including SARS-CoV-2, may be successfully treated with metals (in particular, noble metals) and their complexes. Biomaterials based on metals such as Ag, Cu, gold (Au) and Zn have unique antiviral activities, durability, and efficacy at low concentrations. They are characterized by large-spectrum usage, and can successfully overcome the limitations faced by other conventional medicines since their action is independent of age and comorbidity, no drug resistance is developed, and low cytotoxicity is found[27][28]. However, their antiviral mechanisms are still not completely clear because selective interactions between metals and viral macromolecules, particularly proteins, cannot be easily identified. Experimental data have shown two main antiviral performances of metals: (i) the ability to prevent the viral infection by inhibiting the entrance of virus within the host system; (ii) the ability to affect processes involved in virus replication[29].
Thanks to the ability to slowly release the metal ions from metal NPs, metal-based materials are recently employed as virus spread control tools, providing long-term protection against viruses. For example, it was demonstrated that the use of these materials as coating agents can strongly reduce virus infectivity for several weeks[30]. In a liquid environment, the transport of the virus particles is much slower compared to that of metal ions going from metal NPs, and thus the antiviral effect from metal ions occurs faster[31].
2.1.1. Silver-Based Nanomaterials
Ag NPs, thanks to their tunable physicochemical properties, easy production routes and remarkable biological effects, including excellent antimicrobial action, are one of the most investigated nanomaterials for biomedicine applications[32]. The recent need for an effective agent against SARS-CoV-2 leads to a growing attention on the Ag NPs’ antiviral activity. As well-known, it is correlated to several mechanisms including interactions with viral envelope and with viral surface proteins (preferentially towards the ones rich in sulfhydryl groups). Other mechanisms regard the interactions with host cell pathways to prevent virus penetration and the interactions with viral factors necessary for virus replication[33][34].
Rodrigues et al.[35] investigated, through a computational approach, the spontaneous interaction among silver ions (Ag+) and five amino acids (glutamate, isoleucine, leucine, threonine, and lysine) present in the structure of the SARS-CoV-2 spike protein. The theoretical studies demonstrated that the interactions between Ag+ and -NH2 groups are more favorable rather than those with -C=O. The negative values of Gibbs free energies and the negative enthalpy energy variation (DH < 0) indicate that the interactions Ag+-amino acids are spontaneous and exothermic.
Jeremiah et al. evaluated the efficiency of Ag NPs with different diameters (2 to 15 nm) necked or capped with polyvinylpyrrolidone. This material was proposed as a virus spread control measure (to be used on inanimate and nonbiological surfaces) against SARS-CoV-2 infection, since it is able to disrupt viral integrity[36]. However, these Ag NPs could also interact with ACE2 receptors and/or intracellular mechanisms.
Merkl et al. have tested the antiviral activity against SARS-CoV-2 of glass and porous filter media (glass fiber filters and FFP3 filters) coated with Ag as well as CuO[31]. The antiviral activity of Ag was significantly higher than that of CuO; in fact, Ag reduced the viral load up to 75% after 5 min and 98% after 120 min instead the viral load using CuO was reduced to 54% and 76% after 30 and 120 min, respectively. One can hypothesize that Ag nanomaterial has a direct antiviral activity releasing Ag+ ions which bind to viral proteins or directly damage membranes, while CuO nanomaterials act through an indirect antiviral action by inducing the generation of reactive oxygen species (ROS).
2.1.2. Gold-Based Nanomaterials
Thanks to their good biocompatibility, poor immunogenicity, and ability to bind biological ligands, Au NPs are interesting candidates as antiviral agents[37]. The antiviral mechanism of Au NPs is based on the ability to prevent virus binding to cell membranes, and on the capability to inhibit virus proliferation. Cirri et al. found that also the organo-gold (III) compound Aubipyc possesses antiviral properties[29]. As evidenced by a computational study, the antiviral action of Aubipyc appeared due to the metalation of suitable metal-coordinating sites on viral proteins, that is the deprotonated forms of cysteine and selenocysteine, evidencing the main role played by the pH of the milieu in determining the occurrence of metalation. Although Aubipyc showed a low selectivity index and so it is not very suitable for in vivo tests, it could find useful applications in the field of viral spread control,
2.1.3. Zinc-Based Nanomaterials
Zn has shown virucidal and antiviral properties[38][39][40]. Recenty, several authors have studied the effectiveness of Zn2+ ions against SARS-CoV-2 and the involved mechanisms[41][42]. Pormohammad et al.[43] used molecular modeling to demonstrate that Zn can bind COVID-19 RdRp (RNA-dependent RNA polymerase) and 3CLpro (3C-like proteinase). This bond influences the folded conformation and/or activity of these viral proteins, modulating viral replication. It was demonstrated that Zn2+ ions are able to bind the catalytic dyad of SARS-CoV-2 Mpro (main protease) via metal coordination bonds with His41 and Cys145 residues[44]. Tao et al. demonstrated the ability of Zn gluconate (a common zinc supplement) to inhibit the proteolytic activity of PLpro (papain like protease) and Mpro[45]. Crystallographic studies revealed two potential Zn2+-binding sites, one in the dyad catalytic center, and the other located on the surface of the protein structure. A lower affinity towards Mpro compared to other SARS-CoV-2 targets, including the ACE2 receptor and SARS-CoV-2 RdRp, was also confirmed studying the interaction of these proteins with ZnO by in silico docking studies, showing this descending order: ACE2 > RdRp > Mpro[46] (Figure 3).
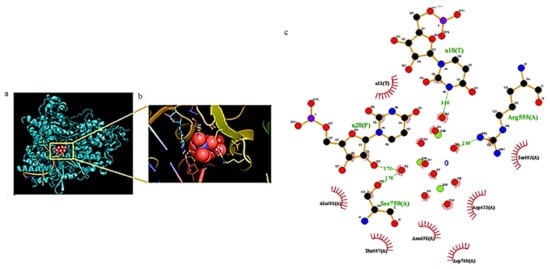
Figure 3. (a) Docking results of ZnO NPs interacting with amino acids of COVID-19 RdRp and (b) corresponding zoom. (c) The related ligand-protein interaction diagrams. The dashed green lines indicate hydrogen bonds. Figure reprinted from Ref.[46] under the terms of the Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) license.
Zn2+ ions embedded in polyamide fibers was projected by Gopal et al. as a hybrid material useful in the fabrication of PPE; this material has the ability to decrease the SARS- CoV-2 titer by approximately 100-fold[41]. Two porous coating materials based on submicrometer ZnO particles bound with silica menisci and on ZnO tetrapods bound with polyurethane were projected by Hosseini et al.; the findings showed that infectivity is strongly related to material porosity and capability to absorb aqueous droplets[42].
Adhikari et al. proved that the favorable interactions between SARS-CoV-2 spike protein, spherical ZnO NPs, and different facets of the ZnO nanostructure induce a denaturation of spike proteins, which may lose the capability to bind the ACE2 receptors in human cells[47]; this non-toxic Zn nanomaterial was duly added to nanoceutical cotton fabric to produce a membrane filter to be employed in face mask fabrication (Figure 4).
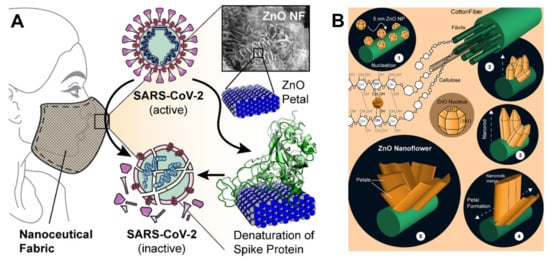
Figure 4. (A) Schematic representation showing how ZnO petals added to nanoceutical cotton fabric may induce a denaturation of the spike protein; (B) The five steps for growing ZnO nanoflowers on cotton cellulose fibers. Reprinted with permission from Adhikari et al. Nanoceutical Fabric Prevents COVID-19 Spread through Expelled Respiratory Droplets: A Combined Computational, Spectroscopic, and Antimicrobial Study. ACS Applied Bio Materials, 2021 4 (7), 5471–5484. Copyright 2021 American Chemical Society[47].
Sportelli et al. verified the anti-SARS-CoV-2 activity of ZnO NPs ecofriendly produced in the presence of both cationic and anionic stabilizers (cetyltrimethylammonium bromide - CTAB, poly-diallyl-(dimethylammonium) chloride - PDDA, poly (sodium 4-styrenesulfonate) - PSS). When tested in vitro, the PDDA-ZnO NPs induced a decrease in viral load between 70% and 90%; however, no contribution was given by PDDA itself to the antiviral activity of the system. Then, PDDA-ZnO NPs were embedded into polyethylene oxide (a biodegradable and nontoxic polymer) film to reach a coating for frequently touched surfaces, with promising results despite the polymeric material limits the active surface of ZnO NPs to exert ionic release and subsequent antiviral activity[48].
A nano-spray disinfectant against SARS-CoV-2 based on ZnO NPs (with a size of about 50 nm) was designed by El-Megharbel et al.[49]. ZnO NPs showed anti-SARS-CoV-2 activity with potent antiviral activity at low concentrations (inhibitory concentrations [IC50] = 526 ng/mL) but with some cytotoxic effect to the cell host (cellular cytotoxicity [CC50] = 292.2 ng/mL), which limits its employment. To overcome the cytotoxicity issue and to reach high performing anti-SARS-CoV-2 systems, the functionalization of ZnO NPs with biocompatible materials (for example, polyethylene glycol) might be an interesting strategy[50].
2.1.4. Copper-Based Nanomaterials
Cu was the first metal to be declared as an effective metallic anti-microbial agent in 2008 by the U.S. Environmental Protection Agency (EPA). Unlike bacteria, viruses do not develop mechanisms of resistance to copper ions, leading to their high susceptibility towards this metal and its derivatives[51]. Antiviral activity of copper compounds seems to depend on the release of Cu2+ ions in solution, which generates ROS, leading to the loss of genome integrity, lipid peroxidation and deactivation of viral enzymes. A contact mechanism, based on metal ion binding and especially effective on the enveloped virus, has been described[52]. However, other mechanisms leading to an antiviral effect cannot be excluded. For example, Almalki et al. have synthetized a new thiazole derivative to inhibit COVID-19, by using Co(II) and Cu(II) complexes[53]. The results of their spectral analysis have been confirmed by theoretical calculations, explaining the details of the interactions with two COVID-19 proteins (namely the structures 6lu7 and 7bz5 from Protein Data Bank). Furthermore, Refat et al.[54] studied, via molecular docking analysis, the interaction of SARS-CoV-2 protease with Cu(II) complexes of deoxycholic acid (used in various fields of human medicine and food industry), evidencing their ability to interact with residues of this target protein.
A computational study by Aallaei et al.[55] demonstrated the correlation between the shape of Cu NPs (to be used as disinfectant) and their interaction with SARS-CoV-2 Mpro and spike glycoprotein. Cylindrical and conical Cu NPs were more efficient than spherical ones.
SARS-CoV-2 inactivation may be also induced by treatment with copper iodide (CuI) NPs[56], to be used as be applied to produce coatings for high-touch surfaces, masks, protective clothing and hand hygiene products. This virucidal action, maintained also using CuI doped film and fabric, is mainly due to the capability to destruct viral spike and nucleocapsid proteins and may be mediated through direct and indirect mechanisms related to cuprous ions (Cu+) release and ROS production. In fact, Cu+ generates hydroxyl radicals both in the presence of H2O2 by a Fenton-like reaction and in the absence of H2O2[57].
Jung et al.[58] showed the excellent antiviral performance of a copper-coated polypropylene (PP) filter face mask prepared by depositing a copper thin film on a spunbond PP filter surrounding a KF94 face mask. Oxygen ion beam pretreatment was employed to improve the film adhesion on the PP fibers and avoid copper film detachment (which should be a significant hazard for the possible inhalation of film particles).
Clay-based materials may be used for different biomedical purposes, due to their interesting properties which include large surface areas and adsorption capacity, thermal and chemical stability, good biocompatibility and low-cost[59]. Materials based on kaolin [Al2Si2O5(OH)4], a 1:1 clay mineral, can retain the virus on their surface, damaging viral proteins and thus blocking cell virus penetration[60]. Rius-Rocabert et al. developed a nanohybrid system consisting of Ag or CuO NPs supported on kaolin plates[61], to be used as a disinfecting antiviral. Both materials showed a strong reduction of viral infectivity against SARS-CoV-2, with a mechanism related to virus adsorption on kaolin plates and virucidal activity of the released metallic NPs and ions Another advantage of this nanocomposite is that kaolinite plates act as a dispenser for the metallic NPs, avoiding a discharge in the environment.
Pan et al. prepared a copper–zinc nanowire (CuZnNW) ink to be sprayed on high-touch surfaces[62]. Cu NW inactivates SARS-CoV-2 faster with respect to bulk Cu, and the addition of a lower amount of Zn (0.16 at % zinc) leads to an improvement of the virucidal activity; Zn stabilizes the copper ions release and thus the coating is effective for a longer time. Zhou et al.[63] have developed novel plastic films finalized to increase the effective contact area between virus particles and the surface coatings and deactivate SARS-CoV-2. The system is based on Ag NPs and Cu NPs (ranging between 10 and 40 nm), combined with nanoscale conical pillars, together with the addition of sodium dodecyl sulfate and polyvinyl acetate to ameliorate the features of the coating deposited on polyethylene terephthalate and polyethylene films (Figure 5).
Figure 5. Simplified scheme about the design of functionalized nanoscale conical pillars with antiviral properties. Figure reused from Ref.[63] under the terms of CC BY 4.0 license.
Bello-Lopez et al. demonstrated the efficacy against SARS-CoV-2 spread of nanometric layers of bimetallic AgCu deposited on polypropylene fibers to prepare reusable cloth masks[64]. Quantum chemistry calculations confirmed that the addition of Ag-Cu NPs makes the polymeric fiber a better electron acceptor, producing damage of viral phospholipids and genetic material. Similarly, Mosselhy et al.[65] showed that two Cu-Ag nanohybrids inhibited SARS-CoV-2 efficiently when used as surface coating; it contained a higher amount of Cu and lower amount of Ag (around 65 and 78 wt% and 7 and 9 wt%, respectively).
2.2 Carbon-Based Nanomaterials
Carbon based nanomaterials thanks to their interesting properties are deeply investigated for several therapeutic applications including antiviral performances[66][67][68][69]. The potentiality as biomedicine tools is mainly ascribed to their capability to cross the cellular membrane by different pathways and to directly interact with several biomolecules such as proteins[70][71].
The high ability of graphene oxide (GO) nanosized sheets to interact with spike protein, ACE2 and the ACE2-bound spike complex was recently studied by molecular docking. The results showed that GO binds strongly ACE2 and spike protein[72]. This is explained taking into account the 12 hydrogen bonds, 2 hydrophobic, and 1 electrostatic interaction computed for ACE2, in comparison with the 7 hydrogen bonds and 2 hydrophobic with ACE2-bound spike complex. GO nanosheets are also capable to hinder virus infectivity, showing in vitro antiviral activity against three different clades of SARS-CoV-2.
The antiviral activity of graphene (G) and GO on Vero cells infected with SARS-CoV-2 was also reported by De Maio et al. Furthermore, in this study, graphene and GO were used to functionalize polyurethane or cotton to obtain new hybrid materials (Figure 6). These latter are able to in vitro eradicate SARS-CoV-2 infectivity and thus potentially useful for the production of PPE[73]. GO could be employed also for water treatment and air purification due to its hydrophilic properties.
Figure 6. Schematic preparation and potentential applications of nanohybrid systems based on (A) hydrophilic GO and (B) hydrophobic graphene materials employed to defeat SARS-CoV-2. Figure reprinted from Ref.[73] under the terms of the Creative Commons CC-BY-NC-ND license.
This entry is adapted from the peer-reviewed paper 10.3390/biom12081060
References
- Mahesh Jayaweera; Hasini Perera; Buddhika Gunawardana; Jagath Manatunge; Transmission of COVID-19 virus by droplets and aerosols: A critical review on the unresolved dichotomy. Environmental Research 2020, 188, 109819-109819, 10.1016/j.envres.2020.109819.
- Michael A. Johansson; Talia M. Quandelacy; Sarah Kada; Pragati Venkata Prasad; Molly Steele; John T. Brooks; Rachel B. Slayton; Matthew Biggerstaff; Jay C. Butler; SARS-CoV-2 Transmission From People Without COVID-19 Symptoms. JAMA Network Open 2021, 4, e2035057-e2035057, 10.1001/jamanetworkopen.2020.35057.
- K. Ravindra; V.S. Malik; B.K. Padhi; S. Goel; M. Gupta; Asymptomatic infection and transmission of COVID-19 among clusters: systematic review and meta-analysis. Public Health 2021, 203, 100-109, 10.1016/j.puhe.2021.12.003.
- David A. Berlin; Roy M. Gulick; Fernando J. Martinez; Severe Covid-19. New England Journal of Medicine 2020, 383, 2451-2460, 10.1056/nejmcp2009575.
- Jerrold H. Levy; Toshiaki Iba; Lyra B. Olson; Kristen M. Corey; Kamrouz Ghadimi; Jean M. Connors; COVID‐19: Thrombosis, thromboinflammation, and anticoagulation considerations. Clinical & Laboratory Haematology 2021, 43, 29-35, 10.1111/ijlh.13500.
- Areez Shafqat; Shameel Shafqat; Sulaiman Al Salameh; Junaid Kashir; Khaled Alkattan; Ahmed Yaqinuddin; Mechanistic Insights Into the Immune Pathophysiology of COVID-19; An In-Depth Review. Frontiers in Immunology 2022, 13, 1-25, 10.3389/fimmu.2022.835104.
- Lavanya Singh; Hendrik Gerhardus Kruger; Glenn Maguire; Thavendran Govender; Raveen Parboosing; The role of nanotechnology in the treatment of viral infections. Therapeutic Advances in Infectious Disease 2017, 4, 105-131, 10.1177/2049936117713593.
- Hamid Rashidzadeh; Hossein Danafar; Hossein Rahimi; Faezeh Mozafari; Marziyeh Salehiabar; Mohammad Amin Rahmati; Samaneh Rahamooz-Haghighi; Navid Mousazadeh; Ali Mohammadi; Yavuz Nuri Ertas; et al. Nanotechnology against the novel coronavirus (severe acute respiratory syndrome coronavirus 2): diagnosis, treatment, therapy and future perspectives. Nanomedicine 2021, 16, 497-516, 10.2217/nnm-2020-0441.
- Yunxiao Feng; Gang Liu; Ming La; Lin Liu; Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles. Molecules 2022, 27, 615, 10.3390/molecules27030615.
- Jong-Woo Lim; Yu-Rim Ahn; Geunseon Park; Hyun-Ouk Kim; Seungjoo Haam; Application of Nanomaterials as an Advanced Strategy for the Diagnosis, Prevention, and Treatment of Viral Diseases. Pharmaceutics 2021, 13, 1570, 10.3390/pharmaceutics13101570.
- Taylor F. Gunnels; Devin M. Stranford; Roxana E. Mitrut; Neha P. Kamat; Joshua N. Leonard; Elucidating Design Principles for Engineering Cell‐Derived Vesicles to Inhibit SARS‐CoV‐2 Infection. Small 2022, 18, 1-14, 10.1002/smll.202200125.
- Lili Liang; Ashiq Ahamed; Liya Ge; Xiaoxu Fu; Grzegorz Lisak; Advances in Antiviral Material Development. ChemPlusChem 2020, 85, 2105-2128, 10.1002/cplu.202000460.
- Jun Lan; Jiwan Ge; Jinfang Yu; Sisi Shan; Huan Zhou; Shilong Fan; Qi Zhang; Xuanling Shi; Qisheng Wang; Linqi Zhang; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215-220, 10.1038/s41586-020-2180-5.
- Qihui Wang; Yanfang Zhang; Lili Wu; Sheng Niu; Chunli Song; Zengyuan Zhang; Guangwen Lu; Chengpeng Qiao; Yu Hu; Kwok-Yung Yuen; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894-904.e9, 10.1016/j.cell.2020.03.045.
- Cody B. Jackson; Michael Farzan; Bing Chen; Hyeryun Choe; Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology 2021, 23, 3-20, 10.1038/s41580-021-00418-x.
- Monica-Carolina Villa-Hermosilla; Sofia Negro; Emilia Barcia; Carolina Hurtado; Consuelo Montejo; Mario Alonso; Ana Fernandez-Carballido; Celecoxib Microparticles for Inhalation in COVID-19-Related Acute Respiratory Distress Syndrome. Pharmaceutics 2022, 14, 1392, 10.3390/pharmaceutics14071392.
- Kirsten Bentley; Richard J. Stanton; Hydroxypropyl Methylcellulose-Based Nasal Sprays Effectively Inhibit In Vitro SARS-CoV-2 Infection and Spread. Viruses 2021, 13, 2345, 10.3390/v13122345.
- Jonathan G.L. Mullins; Drug repurposing in silico screening platforms. Biochemical Society Transactions 2022, 50, 747-758, 10.1042/bst20200967.
- Lifei Ma; Huiyang Li; Jinping Lan; Xiuqing Hao; Huiying Liu; Xiaoman Wang; Yong Huang; Comprehensive analyses of bioinformatics applications in the fight against COVID-19 pandemic. Computational Biology and Chemistry 2021, 95, 107599, 10.1016/j.compbiolchem.2021.107599.
- Mohammad Faheem Khan; Waseem Ahmad Ansari; Tanveer Ahamad; Mohsin Ali Khan; Zaw Ali Khan; Aqib Sarfraz; Mohd Aamish Khan; Bioactive components of different nasal spray solutions may defeat SARS-Cov2: repurposing and in silico studies. Journal of Molecular Modeling 2022, 28, 1-16, 10.1007/s00894-022-05213-9.
- Mohammad Al-Sanea; Narek Abelyan; Mohamed Abdelgawad; Arafa Musa; Mohammed Ghoneim; Tarfah Al-Warhi; Nada Aljaeed; Ohoud Alotaibi; Taghreed Alnusaire; Sayed Abdelwahab; et al. Strawberry and Ginger Silver Nanoparticles as Potential Inhibitors for SARS-CoV-2 Assisted by In Silico Modeling and Metabolic Profiling. Antibiotics 2021, 10, 824, 10.3390/antibiotics10070824.
- Annika Kiel; Bernhard Peter Kaltschmidt; Ehsan Asghari; Andreas Hütten; Barbara Kaltschmidt; Christian Kaltschmidt; Bacterial Biofilm Formation on Nano-Copper Added PLA Suited for 3D Printed Face Masks. Microorganisms 2022, 10, 439, 10.3390/microorganisms10020439.
- Agung Purniawan; Maria Inge Lusida; Royan Wafi Pujiyanto; Aldise Mareta Nastri; Adita Ayu Permanasari; Alfonsus Adrian Hadikusumo Harsono; Nur Hafidzah Oktavia; Sigit Tri Wicaksono; Jezzy Renova Dewantari; Rima Ratnanggana Prasetya; et al. Synthesis and assessment of copper-based nanoparticles as a surface coating agent for antiviral properties against SARS-CoV-2. Scientific Reports 2022, 12, 1-8, 10.1038/s41598-022-08766-0.
- Yao Zhang; Wei Fan; Yanli Sun; Weichun Chen; Yifan Zhang; Application of antiviral materials in textiles: A review. Nanotechnology Reviews 2021, 10, 1092-1115, 10.1515/ntrev-2021-0072.
- Günter Kampf; How long can nosocomial pathogens survive on textiles? A systematic review.. GMS Hyg Infect Control 2020, 15, Doc10, .
- Carsten Weiss; Marie Carriere; Laura Fusco; Ilaria Capua; Jose Angel Regla-Nava; Matteo Pasquali; James A. Scott; Flavia Vitale; Mehmet Altay Unal; Cecilia Mattevi; et al. Toward Nanotechnology-Enabled Approaches against the COVID-19 Pandemic. ACS Nano 2020, 14, 6383-6406, 10.1021/acsnano.0c03697.
- Tabinda Showkat Patoo; Firdous Khanday; Ahsanulhaq Qurashi; Prospectus of advanced nanomaterials for antiviral properties. Materials Advances 2022, 3, 2960-2970, 10.1039/d1ma00541c.
- Cramer, C. Über Oligodynamische Erscheinungen in Lebenden Zellen von C. von Nägeli; Allgemeine Schweizerische Gesellschaft, &c. Neue Denkschriften, &c Tom. XXXIII, livr. 1: 1893. Available online: https://books.google.com.hk/books/about/%C3%9Cber_oligodynamische_Erscheinungen_in_l.html?id=o2xImgEACAAJ&redir_esc=y (accessed on 15 June 2022).
- Damiano Cirri; Tiziano Marzo; Iogann Tolbatov; Alessandro Marrone; Francesco Saladini; Ilaria Vicenti; Filippo Dragoni; Adele Boccuto; Luigi Messori; In Vitro Anti-SARS-CoV-2 Activity of Selected Metal Compounds and Potential Molecular Basis for Their Actions Based on Computational Study. Biomolecules 2021, 11, 1858, 10.3390/biom11121858.
- Maria Chiara Sportelli; Margherita Izzi; Ekaterina A. Kukushkina; Syed Imdadul Hossain; Rosaria Anna Picca; Nicoletta DiTaranto; Nicola Cioffi; Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2?. Nanomaterials 2020, 10, 802, 10.3390/nano10040802.
- Padryk Merkl; Siwen Long; Gerald McInerney; Georgios Sotiriou; Antiviral Activity of Silver, Copper Oxide and Zinc Oxide Nanoparticle Coatings against SARS-CoV-2. Nanomaterials 2021, 11, 1312, 10.3390/nano11051312.
- Oana Gherasim; Rebecca Alexandra Puiu; Alexandra Cătălina Bîrcă; Alexandra-Cristina Burdușel; Alexandru Mihai Grumezescu; An Updated Review on Silver Nanoparticles in Biomedicine. Nanomaterials 2020, 10, 2318, 10.3390/nano10112318.
- Mahendra Rai; Shivaji D. Deshmukh; Avinash P. Ingle; Indarchand Gupta; Massimiliano Galdiero; Stefania Galdiero; Metal nanoparticles: The protective nanoshield against virus infection. Critical Reviews in Microbiology 2014, 42, 46-56, 10.3109/1040841x.2013.879849.
- Jose Luis Elechiguerra; Justin L Burt; Jose R Morones; Alejandra Camacho-Bragado; Xiaoxia Gao; Humberto H Lara; Miguel Jose Yacaman; Interaction of silver nanoparticles with HIV-1. Journal of Nanobiotechnology 2005, 3, 6-6, 10.1186/1477-3155-3-6.
- Jocelia Silva Machado Rodrigues; Aldimar Machado Rodrigues; Divanizia Do Nascimento Souza; Erico Raimundo Pereira de Novais; Alzeir Machado Rodrigues; Glaura Caroena Azevedo de Oliveira; Andrea de Lima Ferreira Novais; DFT calculations to investigate silver ions as a virucide from SARS-CoV-2. Journal of Molecular Modeling 2021, 27, 1-9, 10.1007/s00894-021-04941-8.
- Sundararaj S. Jeremiah; Kei Miyakawa; Takeshi Morita; Yutaro Yamaoka; Akihide Ryo; Potent antiviral effect of silver nanoparticles on SARS-CoV-2. Biochemical and Biophysical Research Communications 2020, 533, 195-200, 10.1016/j.bbrc.2020.09.018.
- Abouzar Babaei; Seyed Mahmoud Mousavi; Marzie Ghasemi; Neda Pirbonyeh; Masoud Soleimani; Afagh Moattari; Gold nanoparticles show potential in vitro antiviral and anticancer activity. Life Sciences 2021, 284, 119652, 10.1016/j.lfs.2021.119652.
- Faten Farouk; Rania Ibrahim Shebl; Comparing Surface Chemical Modifications of Zinc Oxide Nanoparticles for Modulating their Antiviral Activity against Herpes Simplex Virus Type-1. International Journal of Nanoparticles and Nanotechnology 2018, 4, 1-14, 10.35840/2631-5084/5521.
- Scott A Read; Stephanie Obeid; Chantelle Ahlenstiel; Golo Ahlenstiel; The Role of Zinc in Antiviral Immunity. Advances in Nutrition 2019, 10, 696-710, 10.1093/advances/nmz013.
- Dilina Do Nascimento Marreiro; Kyria Jayanne Clímaco Cruz; Ana Raquel Soares de Oliveira; Jennifer Beatriz Silva Morais; Betânia De Jesus E Silva De Almendra Freitas; Stéfany Rodrigues De Sousa Melo; Loanne Rocha dos Santos; Bruna Emanuele Pereira Cardoso; Thaline Milany Da Silva Dias; Antiviral and immunological activity of zinc and possible role in COVID-19. British Journal of Nutrition 2021, 127, 1172-1179, 10.1017/s0007114521002099.
- Vikram Gopal; Benjamin E. Nilsson-Payant; Hollie French; Jurre Y. Siegers; Wai-Shing Yung; Matthew Hardwick; Aartjan J. W. Te Velthuis; Zinc-Embedded Polyamide Fabrics Inactivate SARS-CoV-2 and Influenza A Virus. ACS Applied Materials & Interfaces 2021, 13, 30317-30325, 10.1021/acsami.1c04412.
- Mohsen Hosseini; Saeed Behzadinasab; Alex W.H. Chin; Leo L.M. Poon; William A. Ducker; Reduction of Infectivity of SARS-CoV-2 by Zinc Oxide Coatings. ACS Biomaterials Science & Engineering 2021, 7, 5022-5027, 10.1021/acsbiomaterials.1c01076.
- Ali Pormohammad; Nadia K. Monych; Raymond J. Turner; Zinc and SARS‑CoV‑2: A molecular modeling study of Zn interactions with RNA‑dependent RNA‑polymerase and 3C‑like proteinase enzymes. International Journal of Molecular Medicine 2020, 47, 326-334, 10.3892/ijmm.2020.4790.
- Love Panchariya; Wajahat Ali Khan; Shobhan Kuila; Kirtishila Sonkar; Sibasis Sahoo; Archita Ghoshal; Ankit Kumar; Dileep Kumar Verma; Abdul Hasan; Mohd Azeem Khan; et al. Zinc2+ ion inhibits SARS-CoV-2 main protease and viral replication in vitro. Chemical Communications 2021, 57, 10083-10086, 10.1039/d1cc03563k.
- Xuan Tao; Lu Zhang; Liubing Du; Kai Lu; Zhennan Zhao; Yanxuan Xie; Xiaobo Li; Shuxiang Huang; Pei-Hui Wang; Ji-An Pan; et al. Inhibition of SARS-CoV-2 replication by zinc gluconate in combination with hinokitiol. Journal of Inorganic Biochemistry 2022, 231, 111777, 10.1016/j.jinorgbio.2022.111777.
- Mohamed Hamdi; Hend Mohamed Abdel-Bar; Enas Elmowafy; Ahmed El-Khouly; Mai Mansour; Gehanne A.S. Awad; Investigating the Internalization and COVID-19 Antiviral Computational Analysis of Optimized Nanoscale Zinc Oxide. ACS Omega 2021, 6, 6848-6860, 10.1021/acsomega.0c06046.
- Aniruddha Adhikari; Uttam Pal; Sayan Bayan; Susmita Mondal; Ria Ghosh; Soumendra Darbar; Tanusri Saha-Dasgupta; Samit Kumar Ray; Samir Kumar Pal; Nanoceutical Fabric Prevents COVID-19 Spread through Expelled Respiratory Droplets: A Combined Computational, Spectroscopic, and Antimicrobial Study. ACS Applied Bio Materials 2021, 4, 5471-5484, 10.1021/acsabm.1c00238.
- Maria Chiara Sportelli; Margherita Izzi; Daniela Loconsole; Anna Sallustio; Rosaria Anna Picca; Roberto Felici; Maria Chironna; Nicola Cioffi; On the Efficacy of ZnO Nanostructures against SARS-CoV-2. International Journal of Molecular Sciences 2022, 23, 3040, 10.3390/ijms23063040.
- Samy El-Megharbel; Mohammed Alsawat; Fawziah Al-Salmi; Reham Hamza; Utilizing of (Zinc Oxide Nano-Spray) for Disinfection against “SARS-CoV-2” and Testing Its Biological Effectiveness on Some Biochemical Parameters during (COVID-19 Pandemic)—”ZnO Nanoparticles Have Antiviral Activity against (SARS-CoV-2)”. Coatings 2021, 11, 388, 10.3390/coatings11040388.
- Hadi Ghaffari; Ahmad Tavakoli; Abdolvahab Moradi; Alijan Tabarraei; Farah Bokharaei-Salim; Masoumeh Zahmatkeshan; Mohammad Farahmand; Davod Javanmard; Seyed Jalal Kiani; Maryam Esghaei; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. Journal of Biomedical Science 2019, 26, 1-10, 10.1186/s12929-019-0563-4.
- Brittany S. Mertens; Matthew D. Moore; Lee-Ann Jaykus; Orlin D. Velev; Efficacy and Mechanisms of Copper Ion-Catalyzed Inactivation of Human Norovirus. ACS Infectious Diseases 2022, 8, 855-864, 10.1021/acsinfecdis.1c00609.
- Marin Vincent; Raphaël E. Duval; Philippe Hartemann; Marc Engels‐Deutsch; Contact killing and antimicrobial properties of copper. Journal of Applied Microbiology 2018, 124, 1032-1046, 10.1111/jam.13681.
- Samira A. Almalki; Tahani M. Bawazeer; Basim Asghar; Arwa Alharbi; Meshari M. Aljohani; Mohamed E. Khalifa; Nashwa El-Metwaly; Synthesis and characterization of new thiazole-based Co(II) and Cu(II) complexes; therapeutic function of thiazole towards COVID-19 in comparing to current antivirals in treatment protocol. Journal of Molecular Structure 2021, 1244, 130961, 10.1016/j.molstruc.2021.130961.
- Moamen S. Refat; Safyah B. Bakare; Tariq A. Altalhi; Kehkashan Alam; Ghaferah H. Al-Hazmi; Synthesis and spectroscopic interpretations of Co(II), Ni(II) and Cu(II) decxycholate complexes with molecular docking of COVId-19 protease. Polish Journal of Chemical Technology 2021, 23, 54-59, 10.2478/pjct-2021-0017.
- Mohammadreza Aallaei; Elaheh Molaakbari; Paridokht Mostafavi; Navvabeh Salarizadeh; Rahime Eshaghi Maleksah; Dariush Afzali; Investigation of Cu metal nanoparticles with different morphologies to inhibit SARS-CoV-2 main protease and spike glycoprotein using Molecular Docking and Dynamics Simulation. Journal of Molecular Structure 2021, 1253, 132301, 10.1016/j.molstruc.2021.132301.
- Yohei Takeda; Dulamjav Jamsransuren; Sachiko Matsuda; Roberto Crea; Haruko Ogawa; The SARS-CoV-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (HIDROX®) and Its Use as a Virucidal Cream for Topical Application. Viruses 2021, 13, 232, 10.3390/v13020232.
- Reactions of low-valent transition-metal complexes with hydrogen peroxide. Are they . , , , .
- Sunghoon Jung; Jun-Yeoung Yang; Eun-Yeon Byeon; Do-Geun Kim; Da-Gyum Lee; Sungweon Ryoo; Sanggu Lee; Cheol-Woong Shin; Ho Jang; Hyo Kim; et al. Copper-Coated Polypropylene Filter Face Mask with SARS-CoV-2 Antiviral Ability. Polymers 2021, 13, 1367, 10.3390/polym13091367.
- Marina Massaro; Renato Noto; Serena Riela; Past, Present and Future Perspectives on Halloysite Clay Minerals. Molecules 2020, 25, 4863, 10.3390/molecules25204863.
- Mahmoud E. Awad; Alberto Lopez Galindo; Massimo Setti; Mahmoud M. El-Rahmany; César Viseras Iborra; Kaolinite in pharmaceutics and biomedicine. International Journal of Pharmaceutics 2017, 533, 34-48, 10.1016/j.ijpharm.2017.09.056.
- Sergio Rius-Rocabert; Javier Arranz-Herrero; Adolfo Fernández-Valdés; Marzia Marciello; Sandra Moreno; Francisco Llinares-Pinel; Jesus Presa; Rubén Hernandez-Alcoceba; Roberto López-Píriz; Ramón Torrecillas; et al. Broad virus inactivation using inorganic micro/nano-particulate materials. Materials Today Bio 2021, 13, 100191, 10.1016/j.mtbio.2021.100191.
- Chaochao Pan; Kruttika S. Phadke; Zheng Li; Gaoyuan Ouyang; Tae-Hoon Kim; Lin Zhou; Julie Slaughter; Bryan Bellaire; Shenqiang Ren; Jun Cui; et al. Sprayable copper and copper–zinc nanowires inks for antiviral surface coating. RSC Advances 2022, 12, 6093-6098, 10.1039/d1ra08755j.
- Yuyang Zhou; Nicola F. Fletcher; Nan Zhang; Jaythoon Hassan; Michael D. Gilchrist; Enhancement of Antiviral Effect of Plastic Film against SARS-CoV-2: Combining Nanomaterials and Nanopatterns with Scalability for Mass Manufacturing. Nano Letters 2021, 21, 10149-10156, 10.1021/acs.nanolett.1c02266.
- J. M. Bello-Lopez; P. Silva-Bermudez; Gina Prado-Prone; A Martínez; Gabriela Ibáñez-Cervantes; Mónica Alethia Cureño-Díaz; L. Rocha-Zavaleta; J. Manzo-Merino; Argelia Almaguer-Flores; C Ramos-Vilchis; et al. Biocide effect against SARS-CoV-2 and ESKAPE pathogens of a noncytotoxic silver–copper nanofilm. Biomedical Materials 2022, 17, 015002, 10.1088/1748-605x/ac3208.
- Dina Mosselhy; Lauri Kareinen; Ilkka Kivistö; Kirsi Aaltonen; Jenni Virtanen; Yanling Ge; Tarja Sironen; Copper-Silver Nanohybrids: SARS-CoV-2 Inhibitory Surfaces. Nanomaterials 2021, 11, 1820, 10.3390/nano11071820.
- Govindasamy Rajakumar; Xiu-Hua Zhang; Thandapani Gomathi; Sheng-Fu Wang; Mohammad Azam Ansari; Govindarasu Mydhili; Gnanasundaram Nirmala; Mohammad A. Alzohairy; Ill-Min Chung; Current Use of Carbon-Based Materials for Biomedical Applications—A Prospective and Review. Processes 2020, 8, 355, 10.3390/pr8030355.
- Daniela Caccamo; Monica Currò; Riccardo Ientile; Elisabetta Verderio; Angela Scala; Antonino Mazzaglia; Rosamaria Pennisi; Maria Musarra-Pizzo; Roberto Zagami; Giulia Neri; et al. Intracellular Fate and Impact on Gene Expression of Doxorubicin/Cyclodextrin-Graphene Nanomaterials at Sub-Toxic Concentration. International Journal of Molecular Sciences 2020, 21, 4891, 10.3390/ijms21144891.
- Sahithi Nimushakavi; Shagufta Haque; Rajesh Kotcherlakota; Chitta Ranjan Patra; Biomedical Applications of Carbon Nanotubes: Recent Development and Future Challenges. Nanoengineering of Biomaterials 2021, Chapter 12, 353-388, 10.1002/9783527832095.ch29.
- Ángel Serrano-Aroca; Kazuo Takayama; Alberto Tuñón-Molina; Murat Seyran; Sk. Sarif Hassan; Pabitra Pal Choudhury; Vladimir N. Uversky; Kenneth Lundstrom; Parise Adadi; Giorgio Palù; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069-8086, 10.1021/acsnano.1c00629.
- Marta D’Amora; Silvia Giordani; Carbon Nanomaterials for Nanomedicine. Carbon Nanomaterials for Nanomedicine 2018, Smart Nanoparticles for Biomedicine, 103-113, 10.1016/b978-0-12-814156-4.00007-0.
- Anna Piperno; Angela Scala; Antonino Mazzaglia; Giulia Neri; Rosamaria Pennisi; Maria Teresa Sciortino; Giovanni Grassi; Cellular Signaling Pathways Activated by Functional Graphene Nanomaterials. International Journal of Molecular Sciences 2018, 19, 3365, 10.3390/ijms19113365.
- Mehmet Altay Unal; Fatma Bayrakdar; Hasan Nazir; Omur Besbinar; Cansu Gurcan; Neus Lozano; Luis M. Arellano; Süleyman Yalcin; Oguzhan Panatli; Dogantan Celik; et al. Graphene Oxide Nanosheets Interact and Interfere with SARS‐CoV‐2 Surface Proteins and Cell Receptors to Inhibit Infectivity. Small 2021, 17, 2101483, 10.1002/smll.202101483.
- Flavio De Maio; Valentina Palmieri; Gabriele Babini; Alberto Augello; Ivana Palucci; Giordano Perini; Alessandro Salustri; Patricia Spilman; Marco De Spirito; Maurizio Sanguinetti; et al. Graphene nanoplatelet and graphene oxide functionalization of face mask materials inhibits infectivity of trapped SARS-CoV-2. iScience 2021, 24, 102788, 10.1016/j.isci.2021.102788.
