Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Blood-brain barrier (BBB) integrity is essential to maintaining brain health. Aging-related alterations could lead to chronic progressive leakiness of the BBB, which is directly correlated with cerebrovascular diseases. Indeed, the BBB breakdown during acute ischemic stroke is critical.
- blood-brain barrier
- ischemic stroke
- nanoparticles
1. Introduction
Nowadays, neurological diseases are considered the leading cause of decrease in life expectancy due to disability and the second cause of death worldwide [1,2,3]. These processes are growing at a higher rate than the rest of the human diseases due to the aging of the population. The distribution of years of life lost due to disability from neurological diseases is age-dependent, increasing notably after 50 years [1]. Contrary to the opinion that the prevalence of neurological diseases is more characteristic of Western and more developed countries, when the years of life lost due to disability are adjusted for age, it can be verified that the distribution is similar across the world, regardless of the level of development [4].
Despite the increase in knowledge and the implementation of more and better health care, mortality from neurological diseases increased by 39% and years of life lost due to disability by 15% between 1990 and 2016 [1]. The introduction of preventive practices, especially the control of arterial hypertension, the change in social attitude towards health care and the inclusion of more effective treatments, have achieved a decrease in mortality and disability due to stroke [5,6]. However, the aging of the population [7] will cause an increase in the incidence of stroke in the coming decades [6,8]. To these dramatic incidences of stroke, we must add 10 million patients in Europe who have developed dementia, of which at least half are of vascular origin [8]. The socioeconomic repercussions of stroke are very significant. In 2010, the cost of strokes in Europe was estimated at EUR 905 billion, 60% of which were direct costs. The human cost for individuals, as well as the social cost for their families and communities, is incalculable.
It is well established that the only pharmacological treatment with proven efficacy in the acute phase of ischemic stroke is reperfusion through systemic fibrinolysis, mechanical thrombectomy, or a combination of both. Although mechanical thrombectomy is the preferred treatment [9], its usefulness is limited because of its application to proximal occlusions of the large arteries (about 10% of patients with ischemic stroke) and the need for centers with specialised staff and technology. On the other hand, the comparison of mechanical thrombectomy alone or associated with systemic fibrinolysis has not shown superiority [10,11]. In addition, intravenous or mechanical reperfusion treatment causes a benefit, assessed as an independent functional status at 3 months, of approximately 50% in patients who receive it, which depends on the type of stroke, the time since the onset of symptoms and the quality of the health facilities, which tends to be associated with the level of development of the country. In the best scenario, the patient is permanently left with some disabling injury. For intracerebral haemorrhage, there is no available pharmacological treatment with demonstrated efficacy [12].
In recent decades, attempts have been made to identify modifiable risk factors in the primary prevention of stroke. There is evidence–largely inconclusive–that has guided the management of patients at risk of developing acute episodes of cerebrovascular disease: encouragement of a healthier lifestyle; control of arterial hypertension, hyperlipidaemia, and diabetes; antiplatelet and anticoagulant treatments; and asymptomatic carotid and vertebral stenosis. However, despite extensive research, little solid evidence exists. The arterial hypertension reduction [13,14] and the new anticoagulants in selected groups of patients with atrial fibrillation [15] have shown clear clinical improvements. The correction of other metabolic risk factors does not provide the same level of evidence [16,17,18,19]. Indeed, the efficacy of arterial hypertension control is limited by low compliance with antihypertensive treatment, which is only a quarter in patients in high-income countries and three-quarters in low- and middle-income countries [20,21]. Another problem related to antihypertensive treatment is the possible association with worsening cognitive impairment in patients with brain aging [22,23].
2. BBB Structure and Function
The BBB is a partially dynamic anatomical-functional structure that allows blood to be separated from the brain differentially in various parts of the brain parenchyma and in its communication with the cerebrospinal fluid (CBF) [24]. The bloodstream carries oxygen, nutrients, growth factors, and hormones that coordinate the functioning of the entire body, as well as the complex immune defense system. At the same time, the bloodstream also removes carbon dioxide and metabolic “waste” products from the central nervous system (CNS) [25]. The vascular tree is made up of arteries, arterioles, and the capillary bed, which is responsible for tissue exchange, and subsequently of venules and veins that allow tissue drainage. The microvascularization provided by the capillaries and the postcapillary venules has exclusive peculiarities in the CNS. The capillary network can be made up of non-fenestrated capillaries with a continuous basement membrane, or capillaries and continuous basement membrane, but fenestrated, or discontinuous capillaries that open gaps between the tissue and blood components [25,26].
The BBB is a unique structure in the entire body, exclusive to the CNS. It is made up of continuous and non-fenestrated capillaries, but which also have additional characteristics that further restrict the exchange between the brain and the vascular space [27,28,29]. The BBB protects the brain from compounds and molecules in the bloodstream by allowing only oxygen, glucose, amino acids, and other essential nutrients to cross it. The transport in the BBB can be due to selective transport or by passive diffusion that depends on adenosine triphosphate (ATP) and other metabolized elements from the blood towards the nervous tissue and vice versa [30]. The BBB does not include the entire CNS, and it has different peculiarities in its relationship with the CBF. It is not found in several areas of the CNS such as in some circumventricular organs, the roof of the third and fourth ventricles, the roof of the dicephalus, and the pineal gland [31,32,33,34].
2.1. BBB Molecular Components: The Neurovascular Unit
The endothelial cells are described as the main cells involved in the structure of the BBB. However, these cells are not by themselves capable of carrying out all the functions that the BBB must perform. Thus, close anatomical and functional contact with the pericytes is required. Both types of cells are surrounded by the basement membrane, constituting a vascular unit (Figure 1). Outside it, the vascular basement membrane is reinforced with a parenchymal matrix where the feet of astrocytes rest, which are accompanied by cells with immune functions, such as microglia and macrophages, and neurons. All this cellular functional group constitutes the neurovascular unit (Figure 2a). Therefore, the normal functioning of the BBB requires an adequate interaction between a luminal component, consisting of specific endothelial cells reinforced by pericytes and a vascular basement membrane, and an abluminal component consisting of an extracellular matrix, astrocytes, microglia, macrophages, and neurons [34,35,36,37].
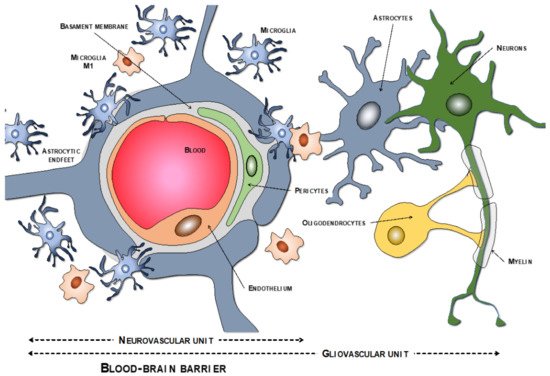
Figure 1. Components of the neurovascular and the gliovascular units of the BBB.
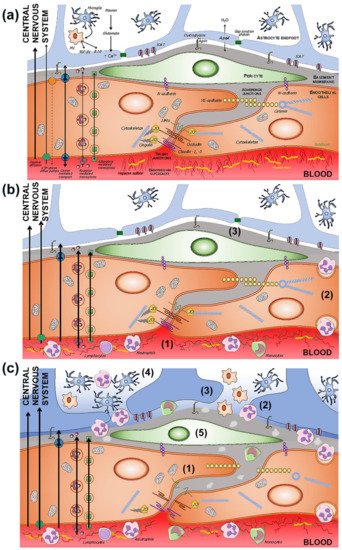
Figure 2. (a) Components of the BBB and the neurovascular unit. The endothelium glycocalyx protects the vascular endothelium and prevents the adhesion of blood cells. The endothelium is impermeable through the paracellular spaces by means of a complex system of proteins that form the tight junctions (TJ). The adherent junctions facilitate the flexibility of the capillary to changes in the diameter of the vascular lumen. Through the endothelium, the passage of molecules necessary for the functioning of the CNS is allowed by various mechanisms, some passive and others linked to exchange or receptor pumps. Pericytes embedded in the basement membrane, astrocytic endfeet, and microglia complete the BBB. (b) BBB dysfunction in situations of chronic and subclinical cerebral ischemia. The increase in permeability is due primarily to the breakdown of the endothelium glycocalyx (1), which facilitates the adhesion and penetration of leukocytes through the endothelium (2), and the alteration of the selective transcellular permeability system, which allows the entry of water and proteins into the perivascular space (3). (c) Hours after the onset of acute cerebral ischemia, tight and adherens junctions break down (1), leukocyte invasion (2), astrocytic endfeet oedema (3), microglia proliferation and activation (4), and pericyte dysfunction (5).
2.2. Transport Pathways across the BBB
The nutritional and metabolic needs of the CNS require that the BBB play a containment role against potentially harmful substances, but at the same time, it should be permeable to other necessary substances. In an intact BBB, this permeability occurs through four physiological mechanisms: passive diffusion, active efflux, transporter-mediated mobility, and receptor-mediated transport. In situations in which the BBB is affected, other mechanisms increase the exchange of substances from the blood to the CNS and vice versa [29].
3. Evolution of BBB Dysfunction in the Setting of Ischemic Stroke
The terms “BBB disruption or BBB breakdown” suggest the destruction of an anatomical barrier resulting in the disappearance of the separation between the vascular component and the CNS parenchyma. However, the BBB is a complex functional system in which cellular elements and extracellular matrices participate with multiple mechanisms that allow both components to become independent and interrelated, and its disruption does not necessarily mean that its constituting anatomical elements have disappeared or been destroyed. As a result, the term "BBB dysfunction" appears more appropriate to describe the changes that occur when any of the mechanisms is altered in a process that can be abrupt at times but can also be dynamic and progressive (Table 1).
Table 1. Summary table of blood-brain barrier components affected by ischemic stroke.
| Blood-Brain Barrier Components |
Change/Event | References |
|---|---|---|
| Glycocalyx | Degradation | [94,95,96,97] |
| Endothelial cells | Interaction with leukocytes Increase in caveolae |
[98,99,100,101,102] |
| Tight junctions | Decrease the expression/ change location of proteins |
[103,104,105,106] |
| Pericytes | Increase in caveolae Astrocytic endfeet oedema |
[102,107,108] |
In rare monogenetic cerebrovascular diseases, the specific alteration of some structural element or some specific function of the BBB may constitute the central element, as in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), Allan-Herndon-Dudley syndrome, Alexander disease, Nasu–Hakkola disease, familial cavernomas, Fahr’s disease, lysosomal storage diseases, and others [28]. However, BBB dysfunction is the cause or consequence of much more frequent neurological processes, such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, dementia associated with human deficiency virus infection (HIV-1), head injuries, ischemic and haemorrhagic stroke, epilepsy, brain tumours, multiple sclerosis, encephalopathies, and encephalitis [24]. An unresolved question is whether BBB dysfunction is the cause, the consequence, or both of many neurological diseases.
In cerebral ischemia, BBB dysfunction can be both the cause and the consequence of increased permeability, as well as the greater or lesser degree of vasogenic oedema or haemorrhagic transformation, associated or not with reperfusion treatments, which characterises the cerebrovascular disease, depending both on the intensity of the ischemia and/or hypoxia, and on the speed of its onset [25,109].
The involvement of the various components of the neurovascular unit is not the same in all ischemic situations. “Chronic” ischemia mainly affects the capillaries of the territories of the penetrating arteries, located in the white matter. In contrast, acute ischemia is the result of the occlusion of much larger caliber arteries. These differences may explain the different mechanisms of BBB dysfunction [86,88,109,110,111,112,113]. The increased permeability of the BBB associated with cerebral ischemia has implicated the structuring of tight and adherent junctions, which would facilitate increased transport of cells, molecules, and solutes through these endothelial intercellular spaces [94,109,111,114,115,116] (Figure 2b). However, other studies have failed to show the alteration of the TJ, either in chronic hypoxia or in the initial phases of acute cerebral ischemia [87,88].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23169486
This entry is offline, you can click here to edit this entry!
