Mesenchymal stem cells (MSCs) have immunomodulatory and regenerative effects in many organs, including the kidney. Emerging evidence has shown that the trophic effects from MSCs are mainly mediated by the paracrine mechanism rather than the direct differentiation of MSCs into injured tissues. These secretomes from MSCs include cytokines, growth factors, chemokines and extracellular vesicles (EVs) containing microRNAs, mRNAs, and proteins. Many research studies have revealed that secretomes from MSCs have potential to ameliorate renal injury in renal disease models, including acute kidney injury and chronic kidney disease through a variety of mechanisms. These trophic mechanisms include immunomodulatory and regenerative effects. In addition, accumulating evidence has uncovered the specific factors and therapeutic mechanisms in MSC-derived EVs. We summarize the immunomodulatory and regenerative effects of EVs from MSCs.
- mesenchymal stem cell
- extracellular vesicles
- microRNA
In addition to the immunomodulatory effects, MSC-EVs have the potential to promote renal regeneration and protection in a variety of mechanisms (Figure 1).
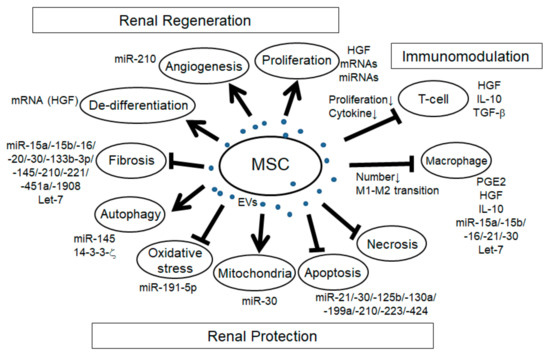
Figure 1. Schema of the effects of mesenchymal stem cell extracellular vesicles (MSC-EVs) in renal injury. Arrows: promotion of the processes; T-bars: inhibition of the processes.
Renal Regeneration
In many reports of AKI models induced by I/R and glycerol and cisplatin-induced rodent models, the increase of tubular proliferation was observed under the treatment of MSC-EVs [17,23,105,115–120], indicating the regenerative effect of MSC-EVs. Although it is not easy to identify the specific factors for cell proliferation, several factors might be involved. For example, HGF has been reported to enhance mitogenesis in I/R-AKI rat model [121]. mRNA and/or miRNA in MSC-EVs might be involved as well since the enhancement of tubular proliferation with MSC-EVs treatment in glycerol-induced AKI was abolished with RNase treatment. Angiogenesis is another mechanism of renal regeneration in rodent AKI models. MSC-EVs treatment in I/R-induced AKI rat model showed the increase of VEGF expression and capillary vessel density that was abolished with RNase treatment [116]. In addition, MSC-EVs treatment in I/R-induced AKI rat model showed the increase of VEGF and VEGFR2 expression as well as the increase of miR-210, and the overexpression of miR-210 in HUVEC-12 cells increased VEGF and VEGFR2 expression and promoted angiogenesis in vitro, indicating that miR-210 might be involved in MSC-EV-induced angiogenesis by targeting VEGF signaling [61]. The expression of angiogenesis indicators, CD31, von Willebrand factor (vWF), and angiopoietin was upregulated with MCS-EVs treatment in I/R-induced AKI rat model [104]. In addition, in the UUO-induced CKD mouse model, MSC-EVs treatment improved the rarefaction of peritubular capillaries detected by CD31 staining [109]. During renal regeneration after AKI, epithelial de-differentiation has been reported to be an important process that promotes cell survival, migration, and proliferation [122]. HGF, TGF-β1, IGF, and EGF have been reported to be involved in the de-differentiation [122,123]. Indeed, Ju et al. reported that MSC-EVs treatment in I/R-induced AKI rat model promoted tubular epithelial cell de-differentiation via HGF induction that was blocked with RNase treatment, suggesting that HGF induction might be mediated by mRNAs and/or miRNAs [124]. Furthermore, they revealed that the HGF mRNA from MSC-EVs entered the injured tubular cells and was translated into HGF protein, indicating foreign HGF synthesis. In summary, MSC-EVs have a variety of trophic mechanisms for renal regeneration through the regulation of cell proliferation, angiogenesis, and tubular cell de-differentiation.
Renal Protection
The mechanism regulating cell apoptosis is one of the most important aspects of renal protection. Indeed, the anti-apoptotic pathway has been reported in many studies with MSC-EVs treatment in a variety of rodent renal injury models, including AKI induced by I/R [24,31,33,104,106,116–118,120,124,125], cisplatin [48,119,126], gentamicin [105], and glycerol [17], hypoxia-induced renal injury [60], aldosterone-induced renal injury [52], and UUO-induced CKD model [109]. Recent advances have gradually uncovered the specific miRNAs, their targets and signaling pathways involved in the effect of anti-apoptosis (Table 2), including PTEN, AKT, mTOR, dynamin-related protein 1 (DRP1), Sema3A, and ERK signaling pathway. Anti-necrosis with MSC-EVs treatment has also been reported in drug-induced AKI, such as with glycerol, cisplatin, and gentamicin [105,115,119]. Autophagy regulation is another aspect of trophic mechanism with MSC-EVs treatment for renal protection. In cisplatin-induced AKI model, the improvement of autophagy was observed with MSC-EVs treatment [14]. In the study, there was an increase of autophagy marker and LC3II expression in vivo and in vitro with MSC-EVs treatment, and it was associated with 14-3-3ζ expression, which regulates ATG-16L. Another group also indicated that MSC-EVs treatment increased the LC3B expression as well as the ATG-5 and ATG-7 gene expressions in normal rat kidney-52E (NRK-52E) cells through the inhibition of mTOR signaling [103]. In addition, Xiang et al. indicated that MSC-EVs enhanced the expression of LC3II and beclin 1 in HK-2 cells through miR-145 targeting PI3K/AKT/mTOR-signaling pathway [32]. Under pathological conditions, autophagy is induced as the adaptive and protective mechanism for cell survival, thus the regulation of autophagy is an important mechanism for renal protection. Preservation of mitochondria is also an important mechanism for renal protection from AKI. DRP1 is known as the key regulator for mitochondrial fission [127] and is rapidly activated after AKI [128]. Inhibition of DRP1 has been reported to protect the kidney from AKI injury caused by I/R and cisplatin [128,129]. Indeed, MSC-EVs treatment preserved mitochondrial function via transferring miR-30 that inhibited the expression of DRP1 [24]. Improvement of oxidative stress with MSC-EVs treatment is also an important mechanism for renal protection against cisplatin and I/R-induced AKI [104,117,119,125]. Oxidative stress via reactive ROS production induces apoptosis, necrosis, and inflammation in AKI. Zhang et al. reported that MSC-EVs treatment alleviated oxidative stress detected by the reduction of malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OhdG) as well as the enhancement of Nrf2 and HO-1 in I/R-induced AKI rat model [125]. NOX-2, known as the inducer of ROS production, declined with MSC-EVs treatment in I/R-induced AKI rat model [117]. In addition, Zhou et al. reported that MSC-EVs treatment ameliorated oxidative stress in cisplatin-induced AKI detected by the reduction of 8-OhdG-positive cells, which reduced the cell apoptosis [119]. HGF has also been reported to improve oxidative stress through the suppression of GLUT1 [130]. Taken together, several factors in MSC-EVs might be involved in the regulation of oxidative stress. Renal fibrosis is the pathological process of CKD, which is strongly associated with the progression of renal dysfunction. The improvement of renal fibrosis with MSC-EVs treatment was reported in a variety of renal injury models, including I/R-induced AKI rodent model [23,104,116,117,124], renovascular stenosis-induced pig CKD model [107], UUO-induced renal fibrosis model [26,28,109], diabetes nephropathy mouse model [29,30], and 5/6 subtotal nephrectomy mouse model [110]. In vitro experiment using HK-2 cells, MSC-EVs ameliorated TGF-β1-induced EMT, which was mediated by miR-133b-3p and miR-294 [27]. As described ahead, let-7c from MSC-EVs ameliorated renal fibrosis in UUO model with the downregulation of Col4a1, MMP-9, TGF-β1, and TGFBR1 [26]. In addition, miR-451 in MSC-EVs ameliorated renal fibrosis by targeting P15 and P19, thus inhibiting EMT in diabetic model [30]. MiR-29b in MSC-EVs might target snail, thereby regulating EMT [57]. Taken together, several MSC-EVs-derived miRNAs have the potential to ameliorate renal fibrosis, mainly through the regulation of TGF-β1-EMT axis. In summary, EV-MSCs have a variety of factors involved in many biological processes for renal generation and protection as well as immunomodulation.
This entry is adapted from the peer-reviewed paper 10.3390/ijms21030756
