1. Ethnobotanical Data of Astragalus Species Used against Cancer
Astragalus mongholicus Bunge is the accepted name of
A. membranaceus var.
mongholicus (Bunge) P.G. Xiao often referred to as
A. membranaceu [
28] is a key plant in Chinese Traditional Medicine used mainly as a Qi (Chi) tonic [
29,
30] but also prescribed against cancer [
30]. A study found that
A. membranaceus is an ingredient in 172 of the 200 analyzed Chinese herbal formulae [
31].
2. Secondary Metabolites of Astragalus Species Anticancer Properties
In recent years, progress in phytochemical studies has been made on
Astragalus species due to their effects as immunostimulants or anticancer agents [
45,
47,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58].
Many
Astragalus species contain cycloartane saponins–astragalosides, which are cycloastragenol derivatives. Some saponins isolated from representatives of the genus are based on an oleanane skeleton [
51,
53]. The high intake of flavonoids is generally associated with a reduced risk of neoplasms [
59,
60]. Over 160 different flavonoids of 90 species of
Astragalus have been isolated and identified as revised previously [
52,
53]. Polysaccharides have been shown to play a role in immune modulation. Astraglucanes have been isolated from roots and rhizomes of
A. mongholicus (syn.
A. membranaceus) [
42] and other species. The polysaccharide fraction contains highly branched, predominantly 1,3-
β-glucans. These products find their application as an aid in radiation antineoplastic therapy and chemotherapy, as well as in the treatment and prevention of bacterial and viral infections [
61].
Different
Astragalus extracts have been shown to increase resistance to the immunosuppressive effects of chemotherapy drugs while stimulating macrophages to produce interleukin-6 and tumor necrosis factor (TNF). Human clinical trials demonstrated a substantial increase in survival rates when extracts from
Astragalus plants are given to cancer patients receiving chemo- or radiotherapies. They have also increased IgA, IgC, and interferon production in humans [
51]. Astragaloside IV inhibited the development of non-small cell lung cancer by inhibiting the Akt/GSK-3p/p-catenin signaling pathway. It also increased the expression of Bax (a cell death marker) while decreasing the expression of Bcl-2 (anti-apoptotic protein). This demonstrates the importance of astragaloside IV as a potential antitumor agent [
62].
A. angustifolius is an endemic Bulgarian species that has been reported to contain cyclosiversigenin (cycloastragenol), siversigenin (astragenol), and soyasapogenol B [
51]. The antiproliferative activity of compounds isolated from
A. angustifolius in cervical (HeLa), human lung (H-446), human colon (HT-29) cancer, and human monocyte lymphoma (U937) cell lines are examined [
63], and only 3-
O-[
α-L-rhamnopyranosyl-(1→2)-
β-D-xylopyranosyl-(1→2)-
β-D-glucuronopyranosyl]-3β,22β,24-trihydroxyolean-12-en-29-oic acid possessed weak cytotoxicity against HeLa.
Another endemic Bulgarian plant is
A. aitosensis which afforded 5,6-dehydro-6-desoxyastragenol [
51].
Recently, a novel and unusual for the genus
Astragalus group of compounds, flavoalkaloids, is identified in
A. monspessulanus subsp.
monspessulanus. Before, they were known only as aglycones (
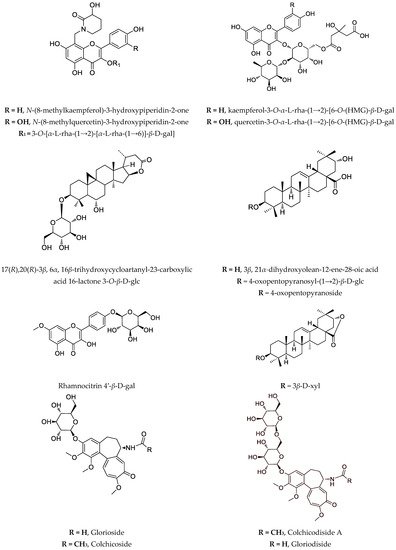
Figure 1). One novel quercetin tetraglycoside and eight known flavonoids are isolated as well [
53]. Also, from the aerial parts of this species, two saponins are reported [
64]. Two rare flavonoids with an unusual hydroxymethylglutaric acid as a moiety: quercetin-3-
O-
α-L-rhamnopyranosyl-(1→2)-[6-
O-(3-hydroxy-3-methylglutaryl)-
β-D-galactopyranoside and kaempferol-3-
O-
α-L-rhamnopyranosyl-(1→2)-[6-
O-(3-hydroxy-3-methylglutaryl)-
β-D-galactopyranoside are isolated from the aerial parts of
A. monspessulanus subsp.
illyricus (
Figure 1) [
65].
Figure 1. Notable compounds, isolated from Astragalus and Gloriosa species.
Phytochemical investigation of
A. glycyphyllos led to the isolation of six saponins, and their structures are partially elucidated [
66,
67]. After acid hydrolysis of a saponin mixture obtained from the aerial parts of the plant, soyasapogenol B and 3β,22β,24-trihidroxyolean-12-en-19-one are identified [
68]. Cycloartane saponins askenoside C and F [
68] and 17
(R), 20
(R)-3
β,6
α,16
β-trihydroxycycloartanyl-23-carboxylic acid 16-lactone 3-
O-
β-D-glucopyranoside are later isolated from the species [
69]. Several known flavonoids, including the rare camelliaside A, are also identified [
53,
69]. The antineoplastic activity in vitro of the saponin-containing fractions obtained from wild-grown and cultivated
A. glycyphyllos, respectively, were tested in a panel of human tumor cell lines of different origin and characteristics. A standard MTT-based protocol for assessing cell viability was used. Both fractions inhibited tumor cell growth in a dose-dependent manner. However, according to the calculated IC
50 value, the fraction obtained from the in vitro shoot cultures showed relatively superior cytotoxic activity compared to that of the wild-type species in all of the screened tumor cell lines (our unpublished data). In vitro cultures of
A. glycyphyllos could be an alternative way to produce saponins, with promising antineoplastic activity.
Three saponins are isolated from
A. corniculatus: two of them with an aglycone 3
β,21
α-dihydoxyolaean-12-ene-28-oic acid, and the third–with its corresponding lactone [
22,
87]. A series of studies demonstrated that a purified saponin fraction containing these compounds had a protective effect against the invasiveness of bone marrow carcinoma (Graffi myeloid tumor) in hamsters. Administration of the saponin mixture increased the number, migration, and phagocytic index of peritoneal macrophages and blood polymorphonuclear leukocytes in animals with implanted tumors. Also, due to the hamster treatment with the mixture, an increased mitogenic response to phytohemagglutinin and lipopolysaccharides is observed, i.e., the saponins have an immunostimulatory effect [
88,
89,
90].
Rhamnocitrin 4′-
β-D-galactopyranoside and a mixture of two saponins are isolated from aerial parts of
A. hamosus and investigated for antiproliferative activity on SKW-3 cells. Significant apoptosis-induction activity is proved for the saponin mixture compared to the flavonoid glycoside at equal concentrations. After co-administration of rhamnocitrin 4′-
β-D-galactopyranoside, with Cisplatin and Gentamicin, there is significant protection of human kidney cells HEK-293T against the cytotoxic effects of nephrotoxic drugs [
75]. The same mixture of two saponins is examined on cell lines HL-60, HL-60/Dox, SKW-3, RPMI-8226, U-266, and OPM-2 [
75]. The saponins caused concentration-dependent suppression of the proliferative activity of malignantly transformed cells. These data are confirmed by an ELISA test evaluating apoptosis-specific DNA fragmentation. The significance of the transcription factor NFκB, as well as the mitochondrial protein Bcl-xL for the antitumor activity of the saponin mixture, is established. Selective cytotoxic activity of saponins in cell lines originating from breast cancer is demonstrated. The saponin mixture showed cytotoxicity concerning both cell lines and clearly demonstrated inhibitory properties against the mitochondrial anti-apoptotic protein Bcl-xL. This gives a reason to believe that unlocking the internal pathway of apoptosis by suppressing the expression of BclxL is a part of the mechanism of action of the saponins. Furthermore, the mixture of two saponins showed no cytotoxic effect on the non-malignant cell line MCF-10A, which originated from the mammary gland, suggesting that it exhibited selective malignant cell toxicity and may be the subject of further studies [
91].
3. Biotechnology of Astragalus Species
Many in vitro cultures are established not only to increase the production of important secondary metabolites in selected
Astragalus plants but also to preserve the endemic and/or endangered species. In general, the most investigated species both in phytochemical and biotechnological means is
A. mongholicus (syn
A. membranaceus) [
92].
3.1. Cell Culture
The active substances from wild and field-grown plants usually have different quality and quantity and vary depending on the environmental conditions. The diseases and the application of pesticides further reduce the quality of the plant material. In vitro plant cultures surmount these problems as environmental conditions affecting the metabolism of plants can be precisely controlled. Working with tissue cells dramatically decreases preparation time, processing, and storage costs associated with traditional plant approaches [
93]. There are several advantages to producing secondary metabolites in plant cell culture compared to in vivo cultivation. Production can be more predictable, reliable, and independent from unpredictably changing climatic conditions. Isolation of the phytochemical metabolites can be more rapid and efficient than extraction from the whole plant. Interfering compounds in the wild plant can be avoided in tissue cultures. Cell cultures can produce phytochemicals in large volumes.
Astragalus genus possesses characteristics that make it significant for in vitro cultivation. Reducing natural supplies due to excessive collection is already present and, therefore, industrial or consumer interest. Due to their complex structures, saponins, flavonoids, and polysaccharides are still most efficiently produced by the plants. There are a lot of problems involved with this production method. Variable qualities and quantities of the plant material, plants that need to grow several years before being ready for harvesting (
Astragalus roots), and the over-collecting of some species (
A. mongholicus, syn.
A. membranaceus),
A. angustifolius,
A. missouriensis,
A. thracicus,
A. aitosensis, etc.) are just a few of the problems connected with the production of these natural products. Therefore, tissue cultures might be explored as an alternative production method [
7].
3.2. Effects of Medium Composition and Growth Regulators
The flavonoid biosynthesis in cell cultures of
A. missouriensis and
A. angustifolius is strongly suppressed by 2,4-dichlorophenoxyacetic acid (2,4-D). The higher concentrations of 2,4-D decreased the content of flavonoids. In this respect, 1-naphthaleneacetic acid (NAA) is weaker than 2,4-D. Therefore, flavonoid production is inhibited by adding 2,4-D to the medium. In all tested concentrations of cytokinins, such as 6-benzylaminopurine (BAP) or kinetin (Kn), under the light cultivation regimen, induction of flavonoid production in cell suspension cultures is achieved. Kinetin was found to be the most effective. The sucrose levels of the medium have a tremendous impact on saponins production. The growth and the saponins production are favored by the higher concentrations of sucrose. The investigation was carried out on
A. membranaceus hairy roots cultures (HR) with different concentrations of sucrose in the MS medium. A basic MS medium supplemented with 2% sucrose increases overall saponins yield but inhibits the growth of HR. The growth of
Astragalus-HR is promoted by high concentration (6%), but the yield of saponins remains very low. The optimal medium for both yield and growth is supplemented with 4% sucrose [
94].
3.3. Effects of End-Product Inhibition
Because phytosterols (campesterol, stigmasterol, and
β-sitosterol) are present in the hairy roots of
Astragalus species, the effect on the total saponin production of these substances is examined. Since the biosynthetic route of saponins and that of phytosterols are branched at 2,3-epoxyscvalen, it may be possible to increase the production of saponins by end-product inhibition. In the experiments, contents of saponins after adding 0.2 mg/mL of
β-sitosterol in MS liquid medium without ammonium nitrate are examined at different stages of growth. The saponin content (total saponins) in the hairy roots induced by
Agrobacterium rhizogenes LBA 9402 reached 5.25% of dry wt on day 28 of cultivation. When
β-sitosterol is added to the culture media of these hairy roots, astragaloside production is remarkably increased to 7.13% of dry wt and led to an increase of 36% of the total saponin content in comparison with the control. From these data,
β-sitosterol seems to behave as an inhibitor in the biosynthetic route when the amount added is relatively large. These results prove that the hairy root cultures of
Astragalus mongholicus can be a valuable alternative for overproducing cycloartane saponins compared with the whole plant. Using a selected high productive clone, inducing by
Agrobacterium rhizogenes LBA 9402, optimized culture medium (MS without ammonium nitrate), and end-product inhibition, a relatively high saponin production can be achieved [
94,
95].
3.4. Genetic Transformation of Astragalus Species by Agrobacterium Rhizogenes
In a specific soil,
A. rhizogenes can induce a certain type of “hairy roots” culture, which can be maintained without phytohormones in the medium [
96]. Usually, four different bacterial strains are used—TR 105, R 1601, ATCC 15834, and LBA 9402. With the same
Agrobacterium strain, the susceptibility of
Astragalus species to infection is highly variable. Some species of the genus (
A. membranaceus, A. mongholicus, A. monspessulanus) have more difficulties establishing transformed roots. In some plants (
A. englerianus, A. mongholicus, A. missouriensis, A. sulcatus), a callus is formed initially, and transformed roots subsequently emerge from it. Still, in others (
A. glycyphyllos, A. hamosus, A. boeticus), a profusion of roots appear directly at the site of inoculation [
70].
3.5. In Vitro Production of Secondary Metabolite
Optimization of cultures and production conditions has been achieved to produce flavonoids from cell cultures of different
Astragalus species:
A. monspessulanus,
A. aitosensis,
A. missouriensis,
A. edulis,
A. hamosus, and
A. angustifolius [
53,
65]. Flavonoids within complex plant tissues can be more difficult to separate in an intact polymeric form than cell culture-derived flavonoids. A novel vehicle for depth investigation of flavonoids individually represents the production of these compounds in uniform plant-cell culture systems.
All in vitro cultures of
A. missouriensis and
A. angustifolius produced flavonoids. Quercetin is the main aglycone identified in the in vitro cell suspension in both free and bound forms (as glycosides). The main flavonoid glycosides are isoquercitrine and quercitrine. Rutin and hyperoside have also been detected. The maximum total amount of flavonoids, 1.78% for
A. angustifolius (unpublished results) and 1.34% for
A. missouriensis, is achieved after optimization of the production medium [
97].
4. Characteristics of Target Gloriosa Species
Genus
Gloriosa (Liliaceae) includes 12 species and, despite its taxonomic complication, was found to be monophyletic [
127]. Few of them are of commercial, pharmaceutical, or ethnobotanical interest.
4.1. Ethnobotaical Data of Gloriosa Species Used against Cancer
Gloriosa superba is one of the plants used as an antidote against snakebite in the Southern part of Tamilnadu, India [
128], and several drops of extract of this plant are rubbed onto the cuts and wounds in Rajouri and Poonch districts of Jammu and Kashmir, India [
129]. It has wide application in folk medicine in tropical Africa and Asia, such as abdominal and general pain, anthelminthic and antiparasitic, leprosy, leucorrhea, mental illness, skin diseases, ulcers, etc. [
130]. But also, in traditional applications in Asia and Africa, in addition to diseases such as gout, scrofula, antipyretic, anthelmintic, purgative, and antiabortive activity, anticancer use is indicated [
131]. This activity is well confirmed in recent pharmacological tests against pancreatic cancer [
79], colon cancer [
84], and other cancer cells [
132]. Due to the boom in harvesting and export trade, some populations of
G. superba are on the edge of extinction [
127].
4.2. Secondary Metabolites of Gloriosa Species with Anticancer Properties
The main secondary metabolite is colchicine, which has anticancer activity but its toxicity profile is not acceptable. Several studies suggested the cytotoxic activity of semisynthetic derivatives of colchicine and thiocolchicoside; thus, the reported IC
50 values have no relevance to naturally occurring tropolones [
133,
134,
135,
136]. Gene expression, as well as cytotoxic effects of colchicine in human gastric cancer ASG and NCI-N87 cell lines, are evaluated. It was found that only 6 ng/mL of colchicine had the desired antiproliferative effect on both lines. Interestingly, the gene regulation of those cells is affected in the same manner as the stated concentration leading to apoptosis [
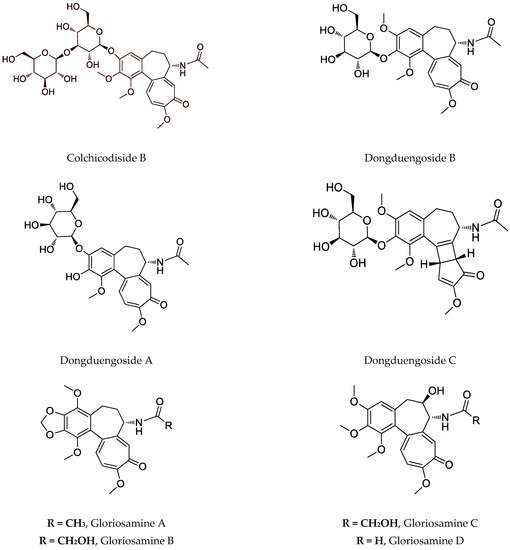
137]. Despite this compound, the interesting colchicinoids such as gloriosamine A-D are isolated from the aerial part of
G. rothschildiana [
86].
Nowadays, the focus on
G. superba is also due to the presence of glycosylated colchicine derivatives, especially colchicoside, which is considered ten times less toxic than colchicine, as shown in
Table 2 [
79]. Recently, four novel colchicinoids named
N-deacetyl-
N-formyl-3-de-
O-methylcolchicine-3-
O-
β-D-glucopyranoside (glorioside), 3-de-
O-methylcolchicine-3-
O-
β-D-glucopyranosyl-(1→6)-3-
O-
β-D-glucopyranoside (colchicodiside A),
N-deacetyl-
N-formyl-3-de-
O-methylcolchicine-3-
O-
β-D-glucopyranosyl-(1→6)-3-
O-
β-D-glucopyranoside (gloriodiside) and 3-de-
O-methylcolchicine-3-
O-
β-D-glucopyranosyl-(1→3)-3-
O-
β-D-glucopyranoside (colchicodiside B) are isolated from the seeds of the species [
81]. Also, from a seedless pot of Thai origin,
G. superba was identified in novel glycosylated colchicinoids–dongduengoside A-C, and colchicine, 2-demethilcolchicine, colchicoside, and luteolin 7-
O-
β-D-glucopyranoside [
82]. Some colchicinoids are obtained using biotechnological approaches. Riva et al. (1997) describe that
β-1,4-galactosyltransferase catalyzes galactosylation of colchicoside, and Pišvejcová et al. (2006) describe the influence of various parameters on the activity of
β-1,4-galactosyltransferase derived from beef milk and the optimization of the conditions leading to the preparation of galactosylate and glycosylated colchicoside derivatives [
138,
139].
4.3. Biotechnology of Gloriosa Species
The medicinal interest in
G. superba and its nonstop over-exploitation are the main reasons to apply in vitro techniques for the conservation, production, and enhancement of secondary metabolites. The species has a very low rate of seed germination as well as seed production is quite low and uneconomical. One of the problems with commercial cultivation is the poor viability of the seeds [
143]. Four or five vegetative cycles are necessary for the complete reproductive phase [
144]. Commercially these plants are propagated using daughter corms with a week multiplication ratio (1:1), slow and insufficient for conservation of this species [
145]. Thus, in vitro cultivation is needed to conserve this taxon, otherwise we will lose it by 2020 [
146]. Plant biotechnological approaches, such as in vitro mass multiplication, have been taken for the conservation, and various methods and techniques have been studied for the production and enhancement of secondary metabolites. An efficient protocol is established for in vitro micro-propagation using corm bud explant [
147]. Extracts from buds inoculated at MS medium supplemented with different concentrations of 2,4-D (1.0–10.0 mg/L) and IAA (0.5–5.0 mg/L) indicated that higher concentrations of 2,4-D and IAA reduce the callus induction. The shoot initiation depends on the combination of cytokinins. Most shoots are obtained in the presence of 9.84 μM 2iP combined with 4.64 μM Kin after 21-day culturing. Sivakumar et al. (2019) developed an efficient protocol for in vitro mass propagation of
G. superba using callus derived from non-dormant corm buds [
148]. Medium supplemented with a combination of plant growth regulators such as BAP (1.5 mg/L), NAA (0.6 mg/L), and polyamine putrescine (15 mg/L) as secondary messengers in signaling pathways, induced maximum shoot buds (87.5). Within this study, optimal seed germination of 86% is also achieved when seeds are treated with 70% sulphuric acid for 2 min. Mahendran et al. (2018) initiated cell suspension cultures of
G. superba with a callus derived from rhizomes cultivated on MS medium supplemented with 2.0 mg/L, 2,4-D, and 0.5 mg/L NAA [
149].
This entry is adapted from the peer-reviewed paper 10.3390/cimb44090267